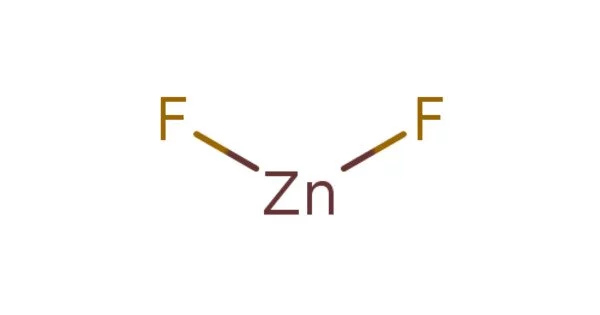
Zinc fluoride (ZnF2) is a chemical compound that is inorganic. It is a white crystalline powder that is used in the production of fluorescent light phosphors. It can be found in both anhydrous and tetrahydrate forms, ZnF2 • 4H2O. (rhombohedral crystal structure). It has a high melting point and a rutile structure with 6 coordinate zinc, indicating a significant ionic character in its chemical bonding. It is less soluble in water than the other zinc halides, ZnCl2, ZnBr2, and ZnI2.
It is also used in electroplating baths, wood preservation, ceramic glazes and enamels, and organic fluorination reactions. ZnF2 crystallizes in the rutile structure, which contains octahedral Zn centers and planar fluorides, like some other metal difluorides.
Properties
Zinc fluoride appears as a white powder or crystalline mass. Its density is 4.84 g / cm3. It is slightly soluble in water and denser than water. It is also insoluble as a hydrate. ZnF2.xH2O. It is used for galvanizing steel and making ceramics.
- Chemical formula: ZnF2
- Molar mass: 103.406 g/mol (anhydrous); 175.45 g/mol (tetrahydrate)
- Appearance: white needles; hygroscopic
- Density: 4.95 g/cm3 (anhydrous); 2.30 g/cm3 (tetrahydrate)
- Melting point: 872 °C (1,602 °F; 1,145 K) (anhydrous); 100 °C, decomposes (tetrahydrate)
- Boiling point: 1,500 °C (2,730 °F; 1,770 K) (anhydrous)
- Solubility in water: .000052 g/100 mL (anhydrous); 1.52 g/100 mL, 20 °C (tetrahydrate)
- Solubility: sparingly soluble in HCl, HNO3, ammonia

Preparation and reactions
Zinc fluoride can be synthesized several ways.
- Reaction of a fluoride salt with zinc chloride, to yield zinc fluoride and a chloride salt, in aqueous solution.
- The reaction of zinc metal with fluorine gas.
- Reaction of hydrofluoric acid with zinc, to yield hydrogen gas (H2) and zinc fluoride (ZnF2).
- Zinc fluoride can be hydrolysed by hot water to form the zinc hydroxyfluoride, Zn(OH)F.
Zinc fluoride may be prepared by heating zinc hydroxide or zinc carbonate with hydrogen fluoride:
Zn(OH)2 + 2HF → ZnF2 + 2H2O
ZnCO3 + 2HF → ZnF2 + CO2 + H2O
Also, it can be precipitated by adding a solution of sodium fluoride to that of zinc acetate:
(CH3COO)2Zn + 2NaF → ZnF2 + 2CH3COONa
Purification Methods
A possible impurity is H2O which can be removed by heating at 100o or by heating to 800o in a dry atmosphere. Heating in the presence of NH4F produces larger crystals. It is sparingly soluble in H2O (1.51g/100mL) but more soluble in HCl, HNO3 and NH4OH. It can be stored in glass bottles.
Application
Zinc fluoride is used in the fluorination of organic compounds, the production of phosphors for fluorescent lights, the preservation of wood, electroplating baths, galvanizing steel, and the production of glazes and enamels in ceramics. It is also used as a termiticide, in pharmaceuticals (for example, to inhibit dentin demineralization and collagen degradation), and as a flux for welding and soldering (e.g. aluminum).