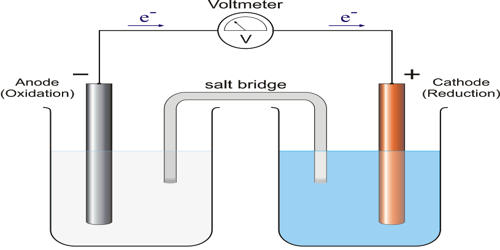
Cathodic protection (CP) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. It is a method for preventing corrosion on submerged and underground metallic structures. A simple method of protection connects the metal to be protected to a more easily corroded “sacrificial metal” to act as the anode. The sacrificial metal then corrodes instead of the protected metal. The technique is based on converting active areas on a metal surface to passive, in other words making them the cathode of an electrochemical cell. It is an electrochemical technique to maintain fully or partially cathodic a metal exposed to an electrolyte, aiming to reduce or even halt corrosion.
It is an electrochemical process that applies DC current to metal to slow or stop corrosion currents. When applied properly, CP stops the corrosion reaction from occurring. For structures such as long pipelines, where passive galvanic cathodic protection is not adequate, an external DC electrical power source is used to provide sufficient current. Cathodic protection is often used to mitigate corrosion damage to active metal surfaces. Cathodic protection is used all over the globe to protect pipelines, water treatment plants, above and underwater storage tanks, ship and boat hulls, offshore production platforms, reinforcement bars in concrete structures and piers, and more.
Types of Cathodic Protection
There are two basic types of cathodic protection: galvanic, and impressed current cathodic protection.
- Galvanic – Galvanic protection consists of applying a protective zinc coating to the steel to prevent rusting. The zinc corrodes in place of the encapsulated steel.
- Impressed Current Cathodic Protection – Impressed current cathodic protection systems consist of anodes that are connected to a power source that provides a perpetual source of electrical flow.
Cathodic protection systems protect a wide range of metallic structures in various environments. In essence, cathodic protection connects the base metal at risk (steel) to a sacrificial metal that corrodes in lieu of the base metal. The technique of providing cathodic protection to steel preserves the metal by providing a highly active metal that can act as an anode and provide free electrons. It is commonly used to protect numerous structures against corrosion, such as ships, offshore floaters, subsea equipment, harbors, pipelines, tanks; basically all submerged or buried metal structures.
Cathodic protection is the application of a DC current onto a metal structure that is in contact with an electrolyte. Common applications are – steel water or fuel pipelines and steel storage tanks such as home water heaters; steel pier piles; ship and boat hulls; offshore oil platforms and onshore oil well casings; offshore wind farm foundations and metal reinforcement bars in concrete buildings and structures. Another common application is in galvanized steel, in which a sacrificial coating of zinc on steel parts protects them from rust.
Cathodic protection can, in some cases, prevent stress corrosion cracking.