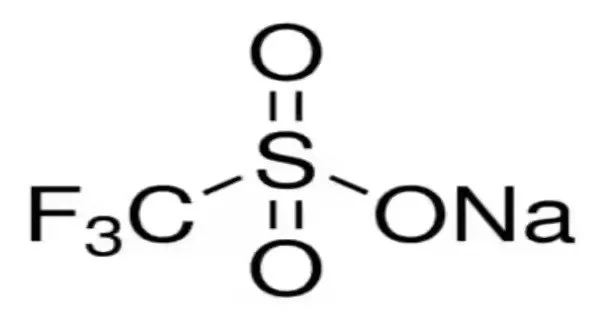
The sodium salt of triflic acid is sodium trifluoromethanesulfinate (CF3SO2Na). It is an organic compound, also known as Langlois’ reagent. This compound is a white solid and is soluble in polar solvents such as water and methanol. This compound was discovered by Langlois to be a suitable reagent for introducing trifluoromethyl groups onto electron-rich aromatic compounds when combined with t-butyl hydroperoxide, an oxidant; this reagent is also known as the Langlois reagent. A free radical mechanism is used in this reaction.
It is a versatile reagent that is widely used in organic chemistry for various reactions. It is often used as a source of the trifluoromethylsulfinyl (CF3SO) group, which is a useful functional group in synthetic chemistry.
Properties
It is a white to pale yellow crystalline solid that is soluble in polar solvents such as water, alcohols, and dimethyl sulfoxide (DMSO). It is an important reagent in modern synthetic organic chemistry due to its versatility and wide range of applications.
- Chemical formula: CF3NaO2S
- Molar mass: 156.05 g·mol−1
- Molecular weight: 190.06 g/mol
- Melting point: 300-302 °C
- Boiling point: Decomposes before boiling
- Density: 1.66 g/cm³
- Solubility: Soluble in water, alcohols, and DMSO, but insoluble in nonpolar solvents such as hexane and benzene.
Under biphasic conditions, this reagent is also capable of trifluoromethylating electron-deficient aromatic compounds. A related polymeric coordination complex, zinc difluoromethanesulfinate, can also introduce difluoromethyl groups (CHF2-) onto aromatic compounds under biphasic conditions.
It provides an environmentally friendly method for the synthesis of β-trifluoromethyl alcohols from alkenes by using DMSO as an oxidant.
Application
One of the most important applications of sodium trifluoromethanesulfinate is in the synthesis of pharmaceuticals, agrochemicals, and materials. It is used as a key building block in the synthesis of various drug molecules, including anti-inflammatory drugs, antiviral drugs, and anticancer agents.
It is also used in the synthesis of other useful compounds, such as trifluoromethylated alkenes, alcohols, and amines. It can be used as a mild oxidizing agent for the conversion of alcohols to aldehydes or ketones. It can also be used in the synthesis of alpha-trifluoromethyl carbonyl compounds, which have important applications in materials science.
Toxicity
Sodium trifluoromethanesulfinate is considered to be relatively non-toxic, but it can cause irritation to the skin, eyes, and respiratory system if inhaled or ingested. Overall, it is a versatile and useful compound in organic chemistry due to its mild reducing properties and solubility in polar solvents.