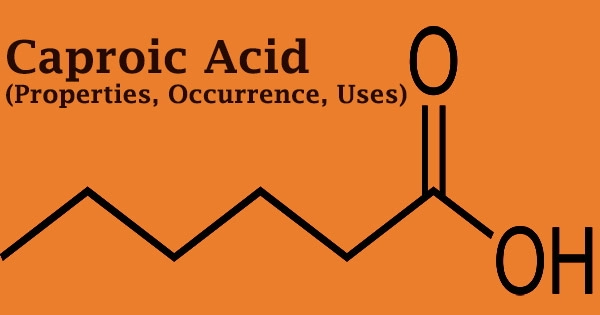
Caproic acid (6 carbon atoms), commonly known as hexanoic acid, is a carboxylic acid produced from hexane with the chemical formula CH3(CH2)4COOH. It gets its name from the Latin word caper, which means “goat.” It is a saturated medium-chain fatty acid with an unpleasant odor that is generated from hexane. Chevreul M.E. was the first to isolate it from butter in 1816.
Caproic acid is a human metabolite as well as a plant metabolite. It’s a colorless, oily liquid with a greasy, cheesy, waxy odor reminiscent of goats or other farm animals. One of its main uses is for the production of esters, which are used to make artificial flavors.
It is a short chain fatty acid (SCFA) with up to 6 carbon atoms that is a saturated fatty acid (no double bond, hence the abbreviation 6:0). It’s also necessary for the production of hexyl derivatives such hexylphenols. Caproic acid is a fatty acid present in a variety of animal fats and oils, and it’s one of the compounds that gives the ginkgo’s disintegrating fleshy seed coat its distinctive flavor.
Hexanoic acid is a type of medium-chain triglyceride (MCT) that is commonly utilized as a dietary supplement in meals, pharmaceuticals, and cosmetics. It’s a hexanoate conjugate acid, and it’s also a component of vanilla and cheese. It is a white oily liquid with a melting point of -3 °C (26.6 °F; 270.15 K) and a boiling point of 205.8 °C (402.44 °F; 478.95 K) at 760 mmHg in pure form.
Caproic acid (or Hexanoic acid) has a bitter taste and an unpleasant odor similar to copra oil. Fractionation of the volatile fatty acids in coconut oil can be used to make this acid. It has an unpleasant odor and appears as a white crystalline solid or a colorless to light yellow solution. In water, it’s insoluble to slightly soluble, and it’s less dense than water.
It smells like copra oil and is disgusting, sweaty, rancid, sour, harsh, pungent, cheesy, fatty, and nasty. Because of his flatulence, he has a foul stench that reminds me of goats, hence his moniker. Caproic acid is primarily used in the production of its esters, which are used as fake flavors, and in the production of hexyl derivatives, such as hexylphenols.
A secondary product of butyric fermentation; found in lavender, camphor, pal marosa, lemongrass, and Juniperus phoenicea essential oils; in a few fruity aromas: apple, currant, and strawberry; also found among the constituents of petitgrain lime oil.
Also reported found in apple, lemon and orange juice, berries, guava, raisin, papaya, peach, pineapple, cooked potato, pepper, breads, cheeses, butter, milk, fish, meats, hop oil, beer, whiskies, rum, brandy, grape wines, coffee, cocoa, tea, filberts, pecans, peanut oil, coconut meat and oil, soybeans, passion fruit, beans, mushrooms, rice, licorice, corn oil, malt, loquat, sherry, clams, mussels, scallops and other sources.
Caproates or hexanoates are the salts and esters of caproic acid. Several progestin medicines, such as hydroxyprogesterone caproate and gestonorone caproate, are caproate esters. Skin, eyes, and mucous membranes may be severely irritated by contact. Ingestion, inhalation, and skin absorption may be hazardous; used to manufacture perfumes.
The material is particularly harmful to mucous membranes, the upper respiratory tract, the eyes, and the skin. As a result of spasm, inflammation, and edema of the larynx and bronchia, chemical pneumonitis, and pulmonary edema, inhalation can be lethal. Caproic acid is rapidly oxidized in mitochondria by beta-oxidation.
Caprylic acid (C8) and capric acid (C10) are two additional acids named for goats. They account for 15% of the fat in goat’s milk, together with caproic acid. Caproic acid is produced by fractional distillation of natural fatty acids or by crude fermentation of butyric acid. Used in the production of rubber chemicals, varnish dryers, resins, and pharmaceuticals.
Caproic, caprylic, and capric acids (capric is a crystal or wax-like substance, whilst the other two are mobile liquids) are often employed “neat” in butter, milk, cream, strawberry, bread, beer, nut, and other flavors, in addition to forming esters. It is possible to create a vast range of items.
Carboxylic acids, like other acids, can initiate polymerization events, and they frequently catalyze (speed up) chemical reactions. Bases, oxidizing agents, and reducing agents all react with hexanoic acid.
Information Sources: