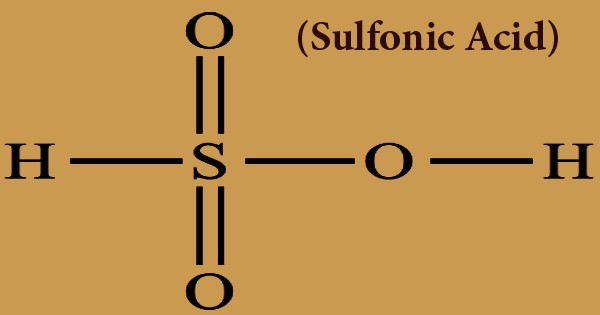
A sulfonic acid (or sulphonic acid) in any class of sulfur-containing organic acids with the general formula R-S(=O)2-OH, in which R is a category of organic combinations. It is classified as a sulfo group, as a substituent. It consists of a sulfur atom, S, attached to a carbon atom that may form part of a large aliphatic or aromatic hydrocarbon, R, and also attached to three oxygen atoms, O, each of which has an attached hydrogen atom, H. A sulfonic acid with one hydroxyl group substituted by an organic substituent may be known to be sulfuric acid. HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. The parent compound (with the organic substituent substituted by hydrogen) is the parent sulfonic acid. Sulfonic acids salts or esters are referred to as sulfonates.
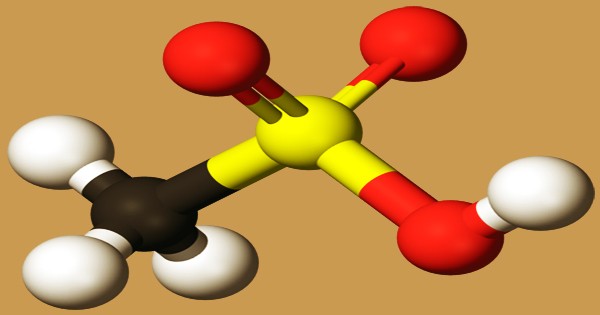
Ball and stick model of methane-sulfonic acid
Some of the most common organosulfur compounds are sulfonic acids; free acids are commonly used in organic synthesis as catalysts, while salts and other derivatives form the basis for the development of detergents, water-soluble dyes and catalysts, pharmaceutical sulfonamides, and ion-exchange resins. Sulfonic acids are formed by the sulfonation process. The sulfonating agent is normally sulfur trioxide. The development of alkylbenzenesulfonic acids is a large-scale application of this process:
RC6H5 + SO3 → RC6H4SO3H
The hydrogen atom makes the compound acidic, just like a carboxylic acid’s hydrogen makes it acidic. However, sulfonic acids are known to be solid acids while carboxylic acids are weak. Methanesulfonic acid into methanedisulfonic acid is also converted by direct sulfonation. Many alkane sulfonic acids are derived from bisulfite, which adds or is alkylated by alkyl halides to terminal alkenes:
HSO3− + RCH=CH2 + H+ → RCH2CH2SO3H
HSO3− + RBr → RSO3H + Br−
As intermediates or starting materials in synthesis, aromatic sulfonic acids are especially useful in the preparation of phenols, for example. Sulfonic acid groups, as seen with the triphenyl phosphine (TPPTS) sulfonic acid derivative, P(C6H4-m-SO3Na)3, can greatly increase the water solubility of compounds. Since sulfonic acids are so acidic, they normally exist as salts and thus appear to be very water-soluble. To stabilize them for use in aqueous dye baths, sulfonic acid groups are also inserted into organic molecules such as dyes. By allowing the dye to bind more closely to the cloth, sulfonic acid groups also enhance the wash fastness of wool and silk dyes.
By hydrolysis of sulfonyl halides and associated precursors, several sulfonic acids are prepared. Perfluorooctanesulfonic acid is thus prepared by sulfonyl fluoride hydrolysis, which is in turn formed by electrofluorination of octanesulfonic acid. Similarly, polyethylene-derived sulfonyl chloride is hydrolyzed into sulphonic acid. These sulfonyl chlorides are manufactured using the Reed reaction by free-radical reactions of chlorine, sulfur dioxide, and hydrocarbons. The metal complexes of this compound are used in industry and in the laboratory as homogeneous catalysts for the synthesis of organic compounds in two-phase systems (for example, in a mixture of water and an organic solvent).
Several sulfonic acids, such as the essential nutrient taurine, occur naturally (2-aminoethanesulfonic acid; NH2CH2CH2SO3H), Sulfobacins and other sulfonolipids (biologically active bacterial products containing 15- to 17-carbon chains bound to 2-aminoethanesulfonic acid carbon and nitrogen) and equinosulfonic acid C (alpha-hydroxysulfonic acid containing two brominated indole rings). Sulfonic acids are acids that are solid. They are widely quoted as being about a million times stronger than the carboxylic acid corresponding to them. P-Toluenesulfonic acid and methanesulfonic acid, for instance, have pKa values of −2.8 and −1.9, respectively, while benzoic acid and acetic acid have values of 4.20 and 4.76, respectively.
The methanesulfonic acid and trifluoromethanesulfonic acid (triflic acid; CF3SO3H) aliphatic sulfonic acids are also commercially important reagents and catalysts. Triflic acid, one of the strongest organic acids known, is used as a catalyst for polymerization and in fuel cells, in the production of gasoline, and in the synthesis of organic and organometallic compounds. In the detergent industry, the most important application of sulfonic acid salts (sulfonates) is as detergents, sodium salts of long-chain aliphatic or aromatic sulfonic acids are used. Unlike ordinary soaps containing carboxylic acid salts, sulfonate-containing soaps do not form scum in hard water because, as with carboxylates, the calcium and magnesium ions present in hard water do not form insoluble sulfonate precipitates.
It is understood that sulfonic acids react to form sodium sulfonate and hydrogen chloride with solid sodium chloride (salt). Sulfonic acids prefer to be crystalline solids or viscous, high-boiling liquids because of their polarity. They are also typically colorless and non-oxidizing, making them suitable in organic reactions for use as acid catalysts. In accordance with their high acidity, their polarity makes water-soluble short-chain sulfonic acids, whereas longer-chain one’s exhibit detergent-like properties. Aromatic sulfonic acids are typically obtained by the treatment of aromatic compounds, called sulfonation, with concentrated sulfuric acid and added sulfur trioxide (‘oleum’).
The oxidation of other organosulfur compounds, the reaction of alkyl halides and metallic sulfites, and the reaction of organometallic compounds with amine complexes of sulfur trioxide (e.g., Me3NSO3) are among the different methods used to prepare aliphatic sulfonic acids. A tetrahedral sulfur center is present in the sulfonic acid group, RSO2OH, indicating that sulfur is at the center of four atoms: three oxygen and one carbon. The sulfur center’s overall geometry is reminiscent of the shape of sulfuric acid. Some derivatives of sulfonic acid, such as sulfa medicinal products, are important as antibiotics. Often useful are polymeric sulfonic acids. Dowex resins are polystyrene sulfonic acid derivatives and are used as catalysts and for ion exchange (water softening). A part of the proton exchange membranes in fuel cells is Nafion, a fluorinated polymeric sulphonic acid.
Information Sources: