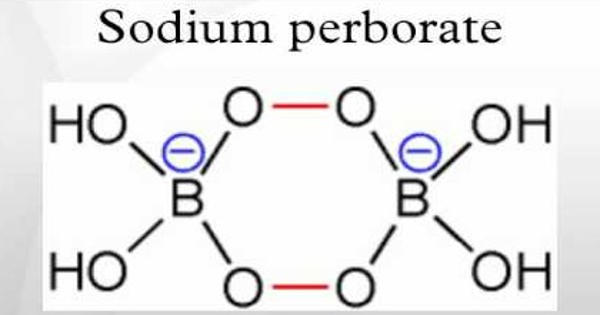
Sodium perborate is an activating and recharging agent for textiles—it converts carboxylate groups of polycarboxylate coatings on cellulose into peroxocarboxylates. It is a chemical compound whose chemical formula may be written NaH2BO4, Na2H4B2O8, or, more properly, [Na+]2·[B2O4(OH)4]2-. Its name is sometimes abbreviated as PBS. It is available inexpensively in large quantities in a fairly pure state—the commercial product is ∼ 96–98% pure. It has a long shelf life and low toxicity.
Sodium perborate is an active form of oxygen used to whiten, brighten, clean, and deodorize. The compound is commonly encountered in anhydrous form or as a hexahydrate (commonly called “monohydrate” or PBS-1 and “tetrahydrate” or PBS-4, after the early assumption that NaBO3 would be the anhydrous form). They are both white, odorless, water-soluble solids.
Sodium perborate may be used in a large number of cleaning products, such as laundry detergents, automatic dishwasher detergents, oxygen powder bleaches, fabric softeners, hand dishwashing detergents, all-purpose cleaners, air fresheners, and stain removers. This salt is widely used in laundry detergents, as one of the peroxide-based bleaches.

Structure
Unlike sodium percarbonate and sodium perphosphate, the compound is not simply an adduct with hydrogen peroxide. Rather, it contains a perborate anion [(B(OH)2OO)2]2- consisting of a cyclic –B–O–O–B–O–O– core with two hydroxy groups attached to each boron atom. The ring adopts a chair conformation.
Pure sodium perborate (NaBO3) is a white, odorless solid under standard conditions. However, a molecule of sodium perborate normally crystallizes with 1, 2 or 4 molecules of water.
Uses
- Sodium perborate serves as a stable source of active oxygen in many detergents, laundry detergents, cleaning products, and laundry bleaches.
- Sodium perborate releases oxygen rapidly at temperatures over 60°C. To make it active at lower temperatures (40–60°C), it has to be mixed with a suitable activator, typically tetraacetylethylenediamine (TAED).
- The compound has antiseptic properties and can act as a disinfectant. It is also used as a “disappearing” preservative in some brands of eye drops.
- Sodium perborate is also used as an oxidizing reagent in organic synthesis. For example, it converts thioethers into sulfoxides and sulfones.
Sodium perborate has also been widely used, though on a much smaller scale, in various dental products, in cosmetics, in cleaning products, in disinfectants, and in decontamination and decommissioning of various organophosphorus compounds.
Information Source: