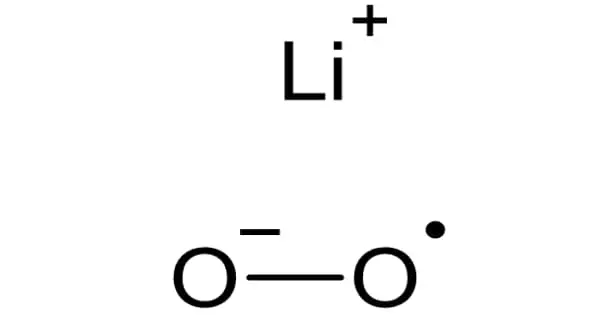Lithium superoxide (LiO2) is an inorganic compound that has only been isolated in matrix isolation experiments at temperatures ranging from 15 to 40 degrees Celsius. Superoxide is a compound that contains the superoxide ion, which has the chemical formula O2–. It is an unstable free radical that has been studied with infrared (IR), Raman, electronic, electron spin resonance, soft X-ray, and theoretical methods.
Structure
Experiments show that the LiO2 molecule contains highly ionic bonds. Using six isotopic species, eighteen different values were obtained. This meant that the force constant between the two oxygen atoms corresponds to the constant discovered for the O2– ion. According to research, the LiO2 molecule has little to no covalent character.
The length of the O-O bond was calculated to be 1.34Å. The Li-O bond was calculated to be approximately 2.1Å0 using a simple crystal structure optimization. Because of the odd electron in the molecular orbital, lithium superoxide is extremely reactive.
There have been numerous studies on the clusters formed by LiO2 molecules. The cage isomer has been discovered to be the most common dimer. The singlet bypyramidal structure comes in second. Studies on the chair complex and the planar ring have also been conducted, but these two are less favorable, if not impossible.
Reactions
In a lithium-air battery, when there is a one-electron reduction during discharge, lithium superoxide is formed as seen in the following reaction:
Li+ + e– + O2 → LiO2
This product will then react and proceed to form lithium peroxide, Li2O2:
2LiO2 → Li2O2 + O2
The mechanism for this last reaction has not been confirmed, and chemists are struggling to develop a theory of what is going on. Another significant challenge for these batteries is identifying an ideal solvent in which to perform these reactions; currently, ether- and amide-based solvents are used, but these compounds readily react with oxygen and decompose. A suitable solvent must be able to resist autoxidation in order for the battery to have a long life cycle.
Applications
The most common application for lithium superoxide is in rechargeable lithium batteries. This lithium compound is a major component as an intermediate, as shown in the reactions above, and this is an area where much research is needed.
The potential energy provided by these batteries has piqued the interest of researchers, with some comparing it to the internal combustion engine. According to one study, alkali superoxides also affect the function of alkyl metals in the atmosphere. The alkali metals are mostly found in the mesosphere, while superoxides are found just below this, where the metal reacts with the excess oxygen.