Carbon Suboxide
Definition
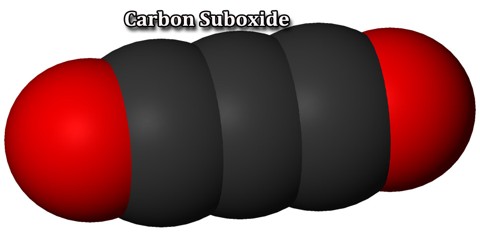
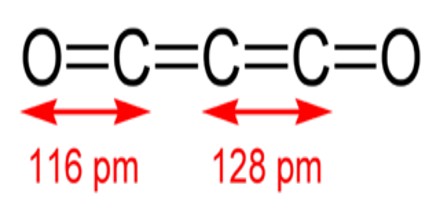
Carbon suboxide is a linear symmetrical molecule whose structure can be represented as O=C=C=C=O. At 25 °C (77 °F) the compound is unstable and polymerizes to highly coloured solid products, but it is a stable molecule at −78 °C (−108.4 °F). It is a foul-smelling lacrimatory (tear-stimulating) gas produced by the dehydration of malonic acid, CH2(COOH)2, with P4O10 in a vacuum at 140 to 150 °C (284 to 302 °F). It is one of the stable members of the series of linear oxocarbons O=Cn=O, which also includes carbon dioxide (CO2) and pentacarbon dioxide (C5O2). Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions.
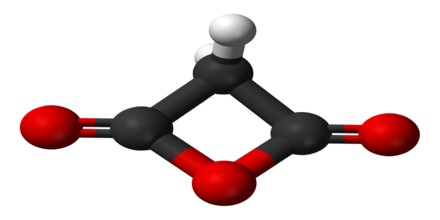
Since carbon suboxide is the acid anhydride of malonic acid, it reacts slowly with water to produce that acid. It is commonly described as an oily liquid or gas at room temperature with an extremely noxious odor.
It is synthesized by warming a dry mixture of phosphorus pentoxide (P4O10) and malonic acid or the esters of malonic acid. Therefore, it can be also considered as the anhydride of malonic anhydride, i.e. the “second anhydride” of malonic acid.
Structure and Uses of Carbon Suboxide
Carbon suboxide, C3O2, is a foul-smelling, lachrymatory gas that can be produced by the dehydration of malonic acid, CH2(COOH)2, with P4O10 in a vacuum at 140° to 150° C. Carbon suboxide is a linear, symmetrical molecule whose structure can be represented as O═C═C═C═O. At 25° C. the compound is unstable and polymerizes to highly-colored solid products, but it is a stable molecule at −78° C. Polymerized carbon suboxide (PCS) is generally considered to be a substance with variable composition as the carbon to oxygen ratio in the PCS is not constant.
A heterocumulene resonance form of carbon suboxide based on minimization of formal charges does not readily explain the molecule’s non-rigidity and deviation from linearity. To account for the quasilinear structure of carbon suboxide, Frenking has proposed that carbon suboxide be regarded as a “coordination complex” of carbon(0) bearing two carbonyl ligands and two lone pairs: OC→:C:←CO. However, the contribution of dative bonding in C3O2 and similar species has been criticized as chemically unreasonable by others.
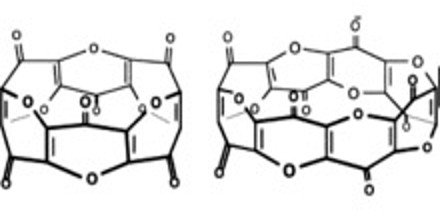
Since carbon suboxide is the acid anhydride of malonic acid, it reacts slowly with water to produce malonic acid. It has been shown that carbon suboxide in an organism can quickly polymerize into macrocyclic polycarbon structures with the common formula (C3O2)n (mostly (C3O2)6 and (C3O2)8), and that those macrocyclic compounds are potent inhibitors of Na+/K+-ATP-ase and Ca-dependent ATP-ase, and have digoxin-like physiological properties and natriuretic and antihypertensive actions.