Tert-butyl alcohol, also known as tert-butanol or t-butanol, is a camphor-like odorless white crystalline solid or colorless liquid (above 77 °F). With the formula (CH3)3COH, it is the most basic tertiary alcohol (sometimes represented as t-BuOH). It is a human xenobiotic metabolite that is formed from an isobutane hydride. It’s one of four butanol isomers that’s miscible with water, ethanol, and diethyl ether. It is miscible with alcohol, ether, and other organic solvents and soluble in water.
tert-butyl alcohol is highly flammable and can be quickly ignited by fire, sparks, or flames; vapors can combine with air to form explosive mixtures. Contact with oxidizing agents, strong mineral acids, or strong hydrochloric acid may result in fire and explosion. Brew and chickpeas have also been found to contain it. It’s also present in cassava, which is used in the fermentation of certain alcoholic beverages. In a fire involving tert-butyl alcohol, toxic gases and vapors (e.g., carbon monoxide and isobutylene) can be emitted.
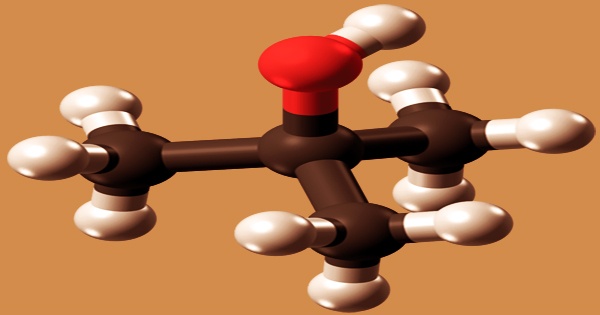
Isobutane, a byproduct of propylene oxide processing, is used to make tert-Butyl alcohol. It can also be made by a Grignard reaction between acetone and methylmagnesium chloride or by catalytic hydration of isobutylene. Dravnieks calculated a detection odor threshold concentration of 2,900 mg/m3 (957 ppmv) in an experiment (1974). Nagata and Takeuchi (1990) recorded a 220 ppbv odor threshold concentration in a later analysis.
Since t-butyl alcohol does not serve as a substrate for alcohol dehydrogenase or catalase’s peroxidative action, it is often used as an example of a non-metabolizable alcohol. Due to the formation of an azeotrope with water, simple distillation cannot be used for purification, though initial drying of a solvent containing large quantities of water can be accomplished by adding benzene to form a tertiary azeotrope and distilling off the water. The isobutane oxidation method for producing propylene oxide produces t-butyl alcohol as a by-product. The acidcatalyzed hydration of isobutylene also produces it.
Drying with calcium oxide (CaO), potassium carbonate (K2CO3), calcium sulfate (CaSO4), or magnesium sulfate (MgSO4), accompanied by fractional distillation, removes smaller quantities of water. Further refluxing and distillation of magnesium activated with iodine, or alkali metals such as sodium or potassium, yields anhydrous tert-butyl alcohol. tert-Butyl alcohol is a radical scavenger that can be oxidized to formaldehyde and acetone in four different ways; (a) iron catalyzed oxidation of ascorbic acid (b) hydrogen peroxide and iron (c) coupled oxidation of xanthine oxidase, an enzymatic bound system (d) NADPH-dependent microsomal electron transfer, a membrane bound system.
Chlorine and alcohols produce alkyl hypochlorites in a similar way. When exposed to sunlight or humidity, they decompose and explode. Secondary and primary hypochlorites are less stable than tertiary hypochlorites. As a solvent, ethanol denaturant, paint remover component, and gasoline octane booster and oxygenate, tert-Butyl alcohol is used. Methyl methacrylate plastics and flotation devices are also made with tert-Butanol. Flavors are made with it, and it’s also used to make artificial musk, fruit essences, and perfume because of its camphor-like fragrance. It can be used for chemical extraction in pharmaceutical applications, as well as coatings on metal and paperboard food containers and industrial cleaning compounds.
T-butyl alcohol can be a useful probe for detecting hydroxyl radicals in intact cells and in vivo due to its unique biochemical properties. The alkoxide is formed by deprotonating tert-Butyl alcohol with a solid base. Potassium tert-butoxide, which is made by treating tert-butanol with potassium metal, is particularly popular.
K + t-BuOH → t-BuO−K+ + 1/2 H2
The toxicity of tert-Butyl alcohol is higher than that of secbutyl alcohol, but lower than that of primary alcohol. Its narcotic effect, however, is greater than that of n-butanol. Inhalation can cause drowsiness and mild skin and eye irritation. Headaches, dizziness, and dry skin can occur after ingestion. It readily abstracts acidic protons from substrates, but its steric bulk prevents it from taking part in nucleophilic substitution reactions like the Williamson ether synthesis or the SN2 reaction.
Resting cell suspensions of methane-grown Methylosinus trichorsporium produced both t-butyl alcohol and isobutyl alcohol after hydroxylation of isobutane at an optimum pH of 6-7 and a temperature of 40 °C. Furthermore, exposure to t-butyl alcohol was linked to a small increase in follicular cell adenoma or thyroid carcinoma (combined) in male mice. On mouse skin, t-butyl alcohol was ineffective as a total carcinogen or a tumor promoter.
O-Chlorination of tert-butyl alcohol with hypochlorous acid to give tert-butyl hypochlorite:
(CH3)3COH + HOCl → (CH3)3COCl + H2O
Strong acids (including mineral acids), strong oxidizers or caustics, aliphatic amines, isocyanates, and alkali metals are incompatible with tert-Butanol (i.e., lithium, sodium, potassium, rubidium, cesium, francium). (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-((2-methylpropan-2-yl)oxy)oxane-2-carboxylic acid and Tert-butyl hydrogen sulfate are two known human metabolites of tert-butanol.
Information Sources: