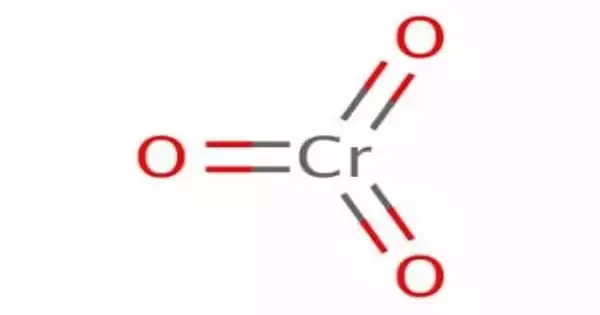
Chromium trioxide has the formula CrO3 and is an inorganic compound. It is the acidic anhydride of chromic acid and is a chromium oxide composed of single chromium bound to three oxygens. It is a strong oxidizing agent that is insoluble in most organic solvents and explodes when exposed to organic compounds and solvents.
It is the acidic anhydride of chromic acid, and it is sometimes marketed as such. This compound is a dark-purple solid when anhydrous, bright orange when wet, and dissolves in water concurrent with hydrolysis. In water, it forms chromic acid and anhydrides, from which commercially available salts such as sodium dichromate (Na2Cr2O7) and pyridinium dichromate are formed. Annually, millions of kilograms are produced, primarily for electroplating. Chromium trioxide is a powerful oxidiser and a carcinogen.
Properties
It is a powerful oxidizer and a suspected carcinogen. It is generally manufactured by treating sodium chromate with sulfuric acid. This compound is a dark-purple solid under anhydrous conditions, bright orange when wet, and which dissolves in water concomitant with hydrolysis.
- Molecular Weight: 99.99
- Appearance: Red to violet crystals
- Melting Point: 196 °C
- Boiling Point: 251 °C
- Density: 2.70 g/cm3
- Solubility in H2O: N/A

Production, structure, and basic reactions
Chromium trioxide is generated by treating sodium chromate or the corresponding sodium dichromate with sulfuric acid:
H2SO4 + Na2Cr2O7 → 2 CrO3 + Na2SO4 + H2O
Approximately 100,000 tonnes are produced annually by this or similar routes.
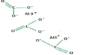
The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center, therefore, shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3.
Applications
Chrome plating is the most common application for chromium trioxide. It is commonly used with additives that have an effect on the plating process but do not react with the trioxide. The trioxide reacts with cadmium, zinc, and other metals to form corrosion-resistant passivating chromate films.
It is also used in the manufacture of synthetic rubies. A chromic acid solution is also used in the application of anodic coatings to aluminum, which is mostly used in aerospace applications.
It is used to control bacteria growth in the wastewater storage tank on the International Space Station. A chromic acid/phosphoric acid solution is also the preferred stripping agent for all types of anodic coatings.
Safety
Chromium trioxide is extremely poisonous, corrosive, and carcinogenic. It is the most common form of hexavalent chromium, which is a known environmental hazard. Because the related chromium(III) derivatives are not particularly hazardous, chromium(VI) samples are destroyed using reductants.
Because chromium trioxide is a strong oxidizer, it will ignite organic materials such as alcohols on contact.