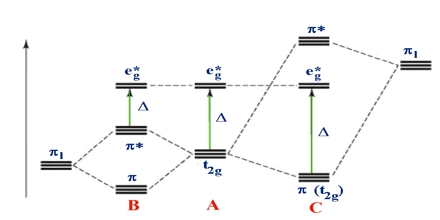
Ligand field theory (LFT) identifies the bonding, orbital agreement, and other characteristics of coordination complexes. It represents a credit card applicatoin of molecular orbital theory to transition metal complexes. A transition metal ion has eight valence atomic orbitals – including things like five nd, 1 (n+1)s, and a few (n+1)p orbitals. These orbitals are usually of appropriate energy to make bonding interaction with ligands.
Ligand Field Theory