Lactobionic acid is classified as a sugar acid. It is a disaccharide formed by oxidizing lactose. It’s a disaccharide made up of gluconic acid and galactose. It is a disaccharide that is formed when beta-D-galactose and D-gluconic acid combine. It functions as an antioxidant. It is derived from lactose. It is a lactobionate conjugate acid.
Lactose oxidation can produce it. Lactobionate is the carboxylate anion of lactobionic acid. Lactobionic acid, as an acid, can form salts with mineral cations like calcium, potassium, sodium, and zinc. Calcium lactobionate is a stabilizer used in food.
Lactobionic acid is hygroscopic and has a high water retention potential, making it suitable for use in cosmetic products. The compound and its constituent mineral salts (Ca, Na, and K lactobionate) are commercially produced for medical and industrial applications, as well as research purposes in some cases. Lactobionic acid is chemically composed of a galactose moiety bonded to a gluconic acid molecule via an ether-like bond. Dehydration of the compound results in the formation of a lactone.
Properties
Lactobionic acid is best described as a natural polyol acid with a formula weight of 358.30 Da and a pKa of 3.8. It is soluble in water as a free acid or as a salt. The molecule possesses multifunctional groups and thus acts as a metal ion chelator and can sequester calcium.
- Melting point: 113-118°C (lit.)
- Boiling point: 410.75°C (rough estimate)
- Density: 1.4662 (rough estimate)
- Solubility: 10 g/100 mL
- Form: Powder
- Color: White to Off-white
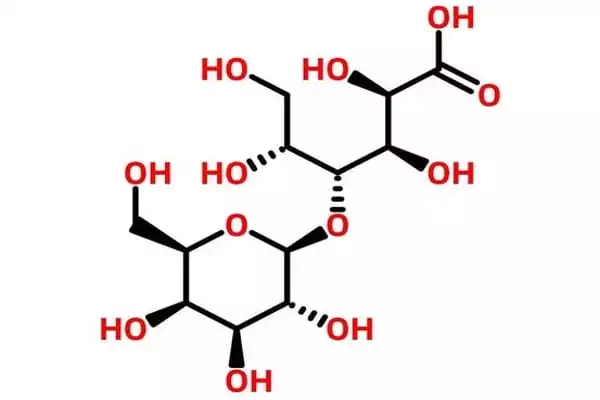
Preparation
The oxidation of the radical aldehyde category of glucose on the lactose molecule to the carboxylic classification is required for the selective transformation of lactose into lactobionic acid. Lactobionic acid is produced through a variety of processes, including enzymatic synthesis, microbial production, biocatalytic oxidation, electrochemical oxidation, and heterogeneous catalytic oxidation.
Extraction and Purification
To increase Lactobionic acid’s productive capacity, the enzymatic reaction can be stopped after several hours of activity and the unchanged substrates can be re-injected into the cycle after the removal of useful products. Because the recovered species are pure, liquid chromatography is the most effective separation method.
By passing the lactobionate-ion solution through a series of ion-transfer resins, a pure solution of Lactobionic acid with minimal amounts of calcium ions is produced. Lactobionic acid can be obtained through a variety of methods including crystallization, evaporation, electrodialysis, and ethanol precipitation.
Uses
Lactobionic acids are commonly found in exfoliating as well as moisturizing products. It is also used as an antioxidant in the cosmetics industry and as an excipient in the pharmaceutical industry. When administered intravenously, the antibiotic erythromycin is administered as the salt erythromycin lactobionate.
Because of its chelating, emulsifying, humectant, and chelating properties, lactobionic acid is widely used in the chemical, food, and pharmaceutical industries. Lactobionic acid and its constituent salts are important food additives due to their excellent solubility, flavor, and health-promoting properties. The compound may also be required for the development of targetable and biocompatible drug delivery systems.