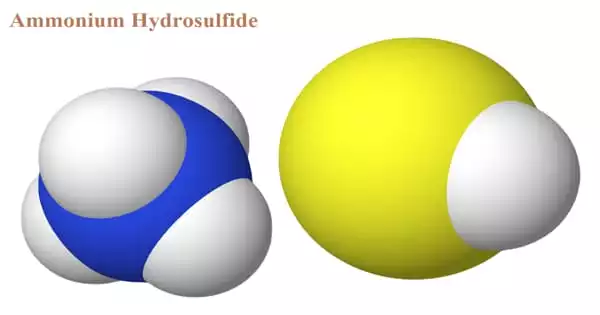
The chemical compound with the formula (NH4)HS is ammonium hydrosulfide. When ammonium hydroxide is treated with an excess of hydrogen sulfide, ammonium hydrosulfide (NH4HS) is generated, which is then treated with the equivalent amount of ammonia to produce ammonium sulfide. The ammonium sulfide formula and its general features will be discussed in this article. The solution of ammonium hydrosulfide is a transparent, yellowish liquid.
Preparation
Ammonium hydrosulfide solutions can be made by passing hydrogen sulfide gas through concentrated ammonia solution. At normal temperature, hydrogen sulfide combines with concentrated aqueous ammonia solution to form (NH4)2S•2NH4HS, according to a thorough 1895 report. When this species is cooled to 0 °C and treated with additional hydrogen sulfide, (NH4)2S•12NH4HS is formed. The hydrosulfide is produced by passing hydrogen sulfide through an ice-cold solution of this material held at 0°C.
The common “stink bomb” consists of an aqueous solution of ammonium sulfide. The mixture easily converts to ammonia and hydrogen sulfide gases. This conversion illustrates the ease of the following equilibrium:
(NH4)SH ⇌ NH3 + H2S
Ammonia and hydrogen sulfide each have a powerful and unpleasant smell.
Composition
It’s a salt made up of ammonium cation and hydrosulfide anion. The salt is found in the form of colorless, water-soluble, micaceous crystals. On Earth, the compound is mostly encountered as a solution, not as a solid, however, NH4SH ice is thought to be a significant component of the cloud decks of the gas-giant planets Jupiter and Saturn, with sulfur created by photolysis being responsible for the hue of certain of those planets’ clouds. It is produced by combining hydrogen sulfide and ammonia.
At room temperature, ammonium sulfide decomposes. It is water-soluble but more soluble in alcohol and extremely soluble in liquid ammonia. Because it is a highly hazardous substance, it must be handled with extreme caution. It is highly caustic in nature and, as previously said, hazardous to the environment. It is known to irritate the skin, eyes, and mucous membranes. The strong odor can make you feel nauseated. Because of its great flammability, it should be kept away from fire and other flammable liquids and chemicals because they can be devastating.
Ammonium polysulphides are another chemical with similar characteristics. Diammonium trisulphide, for example, is an ammonium polysulphide chemical composed of two ammonium ions and three sulphide ions. Hence, the ammonium polysulfide formula will be dependent on the number of sulfide ions present along with the ammonium ions.
Uses
- Used in the manufacture of photographic developers
- Used in the textile industry
- Used in the application of the patina to the bronze