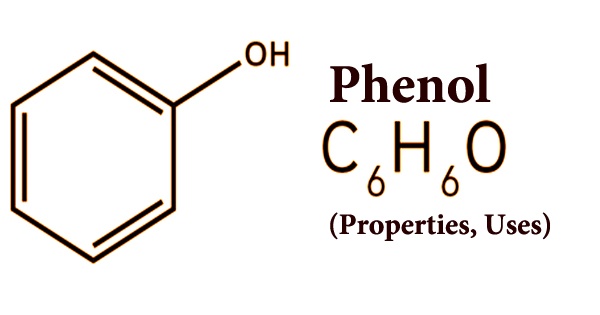
One of a family of organic compounds characterized by a hydroxyl (-OH) group attached to a carbon atom that forms part of an aromatic ring is phenol (also called carbolic acid). Phenol has very active chemical properties and transforms into reddish crystals in the air or in contact with any impurities. The moisture in the air is absorbed and the air is eventually liquefied. Phenol is a synthetic chemical as well as a natural substance; it is a volatile white crystalline solid. Phenol has a distinct odor that is sickeningly sweet and tarry. The consumer product is a liquid. It was first extracted from coal tar, but today it is manufactured from petroleum-derived feedstocks on a large scale (approximately 7 billion kg/year). As a precursor to many products and useful compounds, it is a major industrial product.

Phenol is slightly water-soluble, benzene soluble, alkaline solution, and organic solvents such as ethanol, ether, glycerol, chloroform, etc. It is weakly acidic and reacts to form a salt with base; when dissolved in ferric chloride solution, it appears blue. More slowly than water, phenol evaporates, and a moderate amount can form a solution of water; it can catch fire. The word phenol is also the specific name for its simplest member, monohydroxy benzene (C6H5OH), also known as benzenol, or carbolic acid, in addition to serving as the common name for the whole family.
Phenol is mainly used in the manufacture of phenolic resins and in the processing of nylon and other synthetic fibers. It is also used as a disinfectant and antiseptic in slimicides (chemicals that destroy slime bacteria and fungi), and in medical preparations such as mouthwash and sore throat lozenges. For the production of polycarbonate, epoxy, bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and various pharmaceutical drugs, phenol and its chemical derivatives are important.
In the year 1834, Friedlieb Ferdinand Runge found Phenol; it was extracted from coal tar. Phenol is a remarkably water-soluble organic compound with approximately 84.2 g dissolving in 1000 mL (0.895 M). More than 150 natural products, apricot, sour cherry, black currant, bilberry, cranberry, other berries, grapes, guava fruit, peach, pineapple, asparagus, onion, cooked potato, tomato, cinnamon bark, cassia leaf, ginger, pennyroyal oil, many cheeses, butter, milk, milk powder, boiled egg, fish and fish oil, cooked and cured meats, beer, wheaten bread, crisp bread, cognac, rose wine, cocoa, coffee, tea, whiskies, roasted filbert, roasted peanut, soybean, pecans, honey, avocado, Arctic bramble, passion fruit, beans, mushrooms, burley tobacco, cooked beef and chicken, fermented soy sauce, trassi, roasted almonds, sesame seed, fenugreek, mango, tamarind, Brazil nut, rice, rhubarb, licorice, buckwheat, watercress, malt, wort, dried bonito, loquat, myrtle berry, rosemary, Tahiti and Bourbon vanilla, endive, shrimp, crab, crayfish, clam, squid, truffle and Chinese quince, have been registered.
Phenols have hydroxyl groups that can participate in intermolecular hydrogen bonding similar to alcohols; in fact, phenols appear to form stronger bonds of hydrogen than alcohols. Phenol is an acid that is weak. In aqueous solution in the pH range ca. 8 – 12 it is in equilibrium with the phenolate anion C6H5O− (also called phenoxide):
C6H5OH ⇌ C6H5O− + H+
Phenol is used in the manufacturing or manufacture of explosives, fertilizers, coke, candles, oils, paint removers, rubber, perfumes, asbestos, wood preservatives, synthetic resins, textiles, pharmaceuticals and medicinal products. It is also commonly used in the petroleum, leather, paper, soap, toys, tanning, dye, and agricultural industries as a disinfectant.
Phenol dissolves in water to give a 9.3 percent solution, compared to a 3.6 percent solution in water for cyclohexanol. The interaction between water and phenol is exceptionally strong; it picks up sufficient water from the air to form liquid droplets when crystalline phenol is left out in a humid climate. Phenol has a distinctive odor; a sharp burning taste and it irritates the tissue. It is poisonous not only when swallowed or inhaled, but also when absorbed by the skin. In water, alcohol, and ether, the phenol is soluble. It is used in the manufacture of resins, germicides, weed killers, pharmaceuticals and in the processing of lubricating oil as a solvent.
As the oxygen atoms pi electrons donate electron density into the ring, Phenol is highly reactive to electrophilic aromatic substitution. Through this general approach, through halogenation, acylation, sulphonation, and other methods, several groups can be added to the ring. Most of the phenols used today are made from benzene, either by chlorobenzene hydrolysis or isopropyl benzene oxidation (cumene). The first popular use of phenol was as an antiseptic by Joseph Lister (1827–1912). Infection has frequently resulted in death in human history, even though the wound can be treated surgically. The freezing point is around 105 degrees F. 8.9 lb/gal/density. It is used for the manufacture of plastics, adhesives and other chemicals.
Phenol has been found in the smoke of cigarettes and the emissions of vehicles. Smoke released from a burning mosquito coil (a mosquito repellent) has been found to contain phenol-coated submicron particles and other substances; prolonged exposure can be health-hazardous. Sodium hydroxide forming sodium phenate or phenolate is neutralized, but being weaker than carbonic acid, it cannot be neutralized to release carbon dioxide by sodium bicarbonate or sodium carbonate. For use in cosmetics, Phenol is often considered undesirable. It often causes skin irritation, swelling and rashes, even at low concentrations.
While phenols are not as acidic as carboxylic acids, they are much more acidic than aliphatic alcohols and more acidic than water. Many methods have been developed for its development due to the commercial value of phenol, but only the cumene process is the dominant technology. The main use of phenol today is an effective bactericide for phenolic resins.it, phenol can be used in various consumer goods such as mouthwashes, antiseptic ointments, throat lozenges, air fresheners, eardrops, and lip-balms. Phenol, solid, occurs at 110°F as a solid melt; colorless if pure, pink or red otherwise. Phenol has many other applications, in addition to the building industry. It is used in pharmaceuticals, in pesticides and herbicides, and in paints as a germicide.
Information Sources: