Humanity has a long history of making significant changes without much deliberation. We like inventing solutions to our problems, only to discover that we have produced new ones instead. Therefore, hearing about situations when we’ve paused to think before implementing a radical new concept, like carbon dioxide removal, can be energizing.
Many scientists, environmentalists, and decision-makers have argued in favor of taking steps to directly remove carbon from the atmosphere as carbon emissions continue to rise. They argue that these geoengineering approaches are necessary to avoid catastrophic changes to our land, air and sea.
A suggestion to improve carbon sequestration by raising the ocean’s alkalinity is being examined by researchers at UC Santa Barbara. The goal is to quicken the extremely potent but extremely slow geologic processes that remove carbon from the atmosphere.
The team looked into how this would impact two of the most numerous and significant plankton groups in the ocean, which are at the base of the food chain. Their results, which were reported in Science Advances, imply that the plankton will perform well under the therapy, a finding that stimulates more study into this intriguing idea.
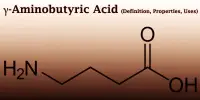
“As we add CO2 into the atmosphere, we’re acidifying the ocean,” said lead author James Gately, a doctoral student at UC Santa Barbara. “Adding alkalinity is essentially like adding an antacid into the ocean.”
Seawater’s chemistry is changed by alkaline or basic substances, which change CO2 into other carbon molecules like carbonate and bicarbonate ions. This lowers the acidity of the water while allowing the ocean to absorb more carbon dioxide.
In fact, the geologic carbon cycle, which recycles carbon between the earth, atmosphere, and ocean over a long period of time, is based on this mechanism. “This process typically takes tens to hundreds of thousands of years to occur,” Gately said. “Our aim is to speed this process up.”
As we add CO2 into the atmosphere, we’re acidifying the ocean. Adding alkalinity is essentially like adding an antacid into the ocean.
James Gately
The driving question for Gately and his colleagues is how will marine life respond to ocean alkalinity enhancement on large scales? They examined the effects of this therapy on diatoms and coccolithophores, two significant kinds of plankton, in order to determine the solution.
Both are significant primary producers that convert sunlight into food and form the foundation of the food chain in the ocean.
“They play a major role in the biological carbon pump, which is essentially the way the oceans lock carbon dioxide away from the atmosphere over millions of years,” said Professor Débora Iglesias-Rodriguez, Gately’s adviser in the Department of Ecology, Evolution, and Marine Biology. These plankton also build exoskeletons, which means they transport large amounts of carbonate, silica, and calcium throughout the biosphere.
Annual phytoplankton blooms (e.g., coccolithophores and diatoms) feed small fish and so forth all the way up the food chain. Following the blooms, the dead cells sink to the ocean floor and produce a sediment that is high in carbonate or silica. This silt gradually traps atmospheric carbon that the organisms had absorbed up via photosynthesis.
Eventually, the seafloor sediment can become chert and limestone. If ocean alkalinity enhancement impacts either of these plankton, the results could be dire.
The team added nutrients and alkalinity to water they collected from the Santa Barbara Channel. Typically, minerals like olivine and various carbonates provide the alkalinity over geologic time, but Gately and his co-authors mimicked this process with other compounds that dissolve and react more quickly.
The water was then sterilized through filtration before bubbling through air that contained 420 ppm (parts per million) of carbon dioxide, which is nearly similar to the CO2 concentrations in today’s atmosphere. After a few days, the team added diatoms and coccolithophores they had cultured in the lab.
The alkalinity of the modern ocean is around 2,300 to 2,400 micro-moles per kilogram of water. The scientists ran one trial at 3,000 µmol/kg, simulating long-term alkalinity addition and another at 5,000 µmol/kg to simulate potential hotspots, like a treatment site.
The authors measured all the physiology, biochemistry, and seawater chemistry of the planktons. Given that the treatment would increase the concentration of calcium ions in the water, they were especially interested in learning whether the coccolithophores would accelerate their calcification.
Ironically, creating calcium carbonate actually produces CO2, even though the compound has carbon and oxygen in it. Coccolithophores are one of the greatest carbon sinks on Earth because, over very long durations, the impacts of sequestration dominate.
Overall, the alkalinity treatments had no discernible effect on the plankton, and calcification remained stable. Even after both treatments, the cells’ photosynthetic efficiency remained far within normal ranges. The authors suspect the decrease may be due to reduced availability of micronutrients, like iron.
In fact, the researchers saw that at increasing alkalinities, dissolved ions precipitated, or changed into solid compounds. This procedure has the potential to remove nutrients and alkalinity from a solution, which could harm marine life and lessen the effectiveness of enhancing ocean alkalinity. The team is already investigating the process in new experiments.
“All told, when we increased alkalinity in the water, the physiology of these organisms did not change,” said Iglesias-Rodriguez. While the results are encouraging, the authors caution against extrapolating out to the ecosystem scale, because responses can vary by species. “Phytoplankton is a good start, but we need to test this in other organisms and ecosystems as well.”
The team has already started conducting experiments on whole plankton populations to increase alkalinity while maintaining natural nutrient concentrations. They’ll measure the response of individual species as well as the community as a whole. Eventually the team plans to take the research out of the lab and into the field.
“It’s very exciting, but we need to operate with caution,” Iglesias-Rodriguez said.
Like many geoengineering proposals, ocean alkalinity enhancement is a controversial topic. “We’re not saying this is a good idea,” Gately said, “we’re trying to determine whether it is or not.”
The problem is that it’s too late to simply rely on reducing our emissions if we want to keep warming under 2 degrees Celsius. Expanding carbon removal to the gigaton scale will require several approaches; ocean alkalinity enhancement is merely one of them.
“None of these technologies is a silver bullet for climate change,” Gately said, “but the ocean sequesters over an order of magnitude more carbon than the land and atmosphere combined, making ocean-focused approaches attractive.”
That said, geoengineering alone can’t solve the problem unless society reduces greenhouse gas emissions. “If we’re in a boat with a hole, we can use a bucket to try to scoop out the water,” Gately added. “But if we don’t plug the hole, we’re going to sink.”