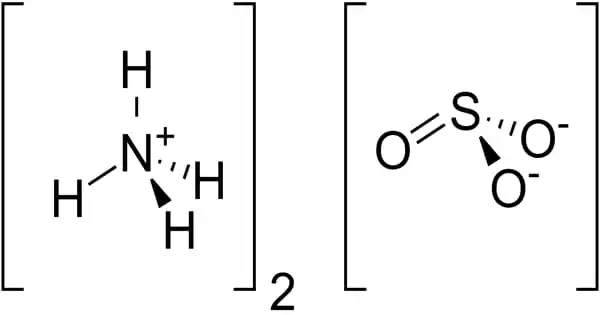
The ammonium salt of sulfurous acid with the chemical formula (NH4)2SO3 is ammonium sulfite. It is a sulfite salt and a p-block molecular entity. It is a crystalline solid with no color. The main risk is the threat to the environment. Immediate action should be taken to limit its environmental spread. It is used in the production of other chemicals, as well as in medicine and photography.
It can be used as an efficient absorbent to remove a small amount of hydrogen sulfide from coal gas, and it can also be used in medicine, as a photographic reducing agent, as a dye intermediate, in paper making, as a food additive, in water treatment, and so on.
Properties
Ammonium sulfite is a colorless to yellow crystalline (sandy or sugary) solid that is typically sold or used in a 40% solution. This salt can be formed by the reaction of ammonia and sulfur dioxide or hydrogen sulfide. This product is commonly found in the overhead of crude distillation units and is highly corrosive to steel equipment.
- Chemical formula: (NH4)2SO3
- Molar mass: 116.14 g/mol
- Appearance: colourless hygroscopic, Crystals
- Melting point: 65 °C (149 °F; 338 K) decomposes
- Solubility in water: 35 g/100 mL; 32.4g/100mL at 0 degrees Celsius
- Solubility: Insoluble in acetone and alcohol

Chemical properties
A reducing agent is ammonium sulfite. When heated to decomposition, it emits sulfur dioxide and nitrogen oxides. Ammonium sulfite has a specific gravity of 1.41. Ammonium sulfite has a refractive index of 1.515.
Lubricants for cold metal working can contain ammonium sulfite. The lubricants are designed to reduce friction in order to reduce heat production and keep impurities out of the metals.
Preparation
Ammonium sulfite can be prepared by the reaction of ammonia with sulfur dioxide in aqueous solution:
2 NH3 + SO2 + H2O → (NH4)2SO3
Ammonium sulfite is made in gas scrubbers, which use ammonium hydroxide to remove sulfur dioxide from power plant emissions. The Walther Process is based on conversion. The resulting ammonium sulfite can be air oxidized to yield ammonium sulfate.
Uses
Ammonium sulfite is the precursor to ammonium thiosulfate via reaction with elemental sulfur. It is used in medicines, metal lubricants, explosives, photography, hair wave solutions, and the production of other chemicals. It is also used as a preservative and to treat agricultural grain.
Ammonium sulfite is used in cosmetics as a hair straightening and waving agent. Due to the damaging nature of sodium hydroxide on hair, ammonium-based hair products have been developed to replace sodium hydroxide-based products.
In photography, ammonium sulfite is used as a fixer preservative. When developing film photographs, one of the reducing agents used to preserve the hypo is ammonium sulfite (sodium thiosulfate or ammonium thiosulfate). Ammonium sulfite is also used in the manufacture of bricks. Ammonium sulfite bricks are primarily used for blast furnace linings.