Cupellation is the process of recovering precious metals from lead by melting the alloy in a cupel and oxidizing the lead by means of an air blast. It is a refining process in metallurgy where ores or alloyed metals are treated under very high temperatures and have controlled operations to separate noble metals, like gold and silver, from base metals, like lead, copper, zinc, arsenic, antimony, or bismuth, present in the ore. It is the separation of gold or silver from impurities by melting the impure metal in a cupel and then directing a blast of hot air on it in a special furnace. The process depends upon the property possessed by the lead of becoming oxidized when strongly heated, while the precious metals are not so affected.
Cupellation is the process of recovering precious metals from lead by melting the alloy in a cupel and oxidizing the lead by means of an air blast. The process is based on the principle that precious metals do not oxidize or react chemically, unlike the base metals, so when they are heated at high temperatures, the precious metals remain apart, and the others react, forming slags or other compounds. The impurities, including lead, copper, tin, and other unwanted metals, are oxidized and partly vaporized and partly absorbed into the pores of the cupel.
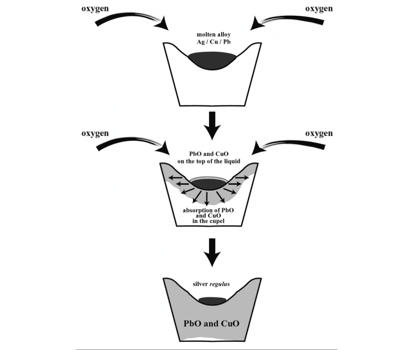
It is the process consist of exposing the cupel containing the metal to be assayed or refined to a hot blast, by which the lead, copper, tin, etc., are oxidized, dissolved, and carried down into the porous cupel, leaving the inoxidizable precious metal. Since the Early Bronze Age, the process was used to obtain silver from smelted lead ores. By the Middle Ages and the Renaissance, cupellation was one of the most common processes for refining precious metals. By then, fire assays were used for assaying minerals, that is, testing fresh metals such as lead and recycled metals to know their purity for jewelry and coin making. Cupellation is still in use today.