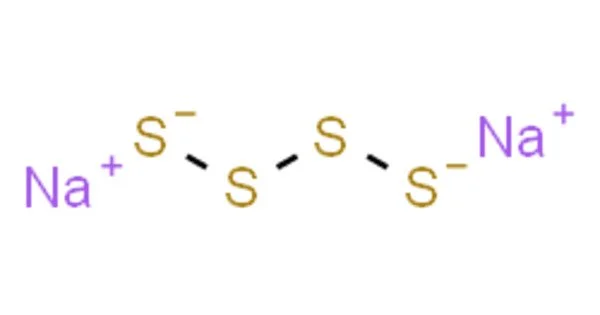Sodium tetrasulfide is an inorganic compound with the formula Na2S4. It is a yellow-orange solid that dissolves via hydrolysis in water. It is soluble in water and ethanol, but insoluble in organic solvents. It is a precursor to some specialty polymers and intermediates in prototypes of the sodium-sulfur battery. It is hygroscopic, meaning it readily absorbs moisture from the air. It is also sensitive to heat and light.
Sodium tetrasulfide is primarily used in the production of sulfur dyes for textiles. It is also used in the manufacturing of rubber and as a laboratory reagent.
Properties
It is a yellow to light brown crystalline solid that is highly soluble in water. It is a strong reducing agent and is often used in organic synthesis as a source of sulfur for thiolation reactions. It is a reducing agent and can be oxidized to sodium sulfate. It reacts with acids to release hydrogen sulfide gas.
- Chemical formula: Na2S4
- Molar mass: 174.24g/mol
- Appearance: Dark red, slightly viscous liquid or yellow crystalline powder
- Density: 1.268 g/cm3 at 15.5 °C
- Melting point: 275 °C (527 °F; 548 K)
- Solubility in water: Soluble in water
Preparation
It can be prepared by treating sodium sulfide with sulfur at high temperature and pressure. The resulting mixture is cooled and the sodium tetrasulfide is separated from unreacted sulfur and sodium sulfide.
Synthesis and structure
It is produced through the reaction between elemental sulfur and sodium hydrosulfide in alcoholic solution:
2NaSH + 4 S → Na2S4 + H2S
The polysulfide anions adopt zig-zag chains of sulfur atoms. The S-S distances are about 2.05 Å and the S-S-S-S dihedral angles are around 90°.
Application
Sodium tetrasulfide is used as a source of sulfur in thiolation reactions, which are widely used in the synthesis of organic compounds such as drugs, agrochemicals, and fine chemicals. It is used as a reducing agent in the production of certain dyes and pigments. It helps to reduce the metal ions in the dye or pigment, resulting in a more stable and uniform product.
Sodium tetrasulfide is also used in the leather industry for dehairing hides and in the production of dyes and pigments. It can be a hazardous material if not handled properly, and exposure to it can cause skin and eye irritation or other health problems.
Hazards
Sodium tetrasulfide can be hazardous if ingested, inhaled, or absorbed through the skin. It can cause skin and eye irritation, respiratory problems, and gastrointestinal issues. It can also release toxic hydrogen sulfide gas if exposed to acids. Proper safety precautions, such as wearing protective clothing and working in a well-ventilated area, should be taken when handling this compound.
However, it is important to handle and use sodium tetrasulfide with caution as it can be a hazardous material. It can cause skin and eye irritation or other health problems if not handled properly.