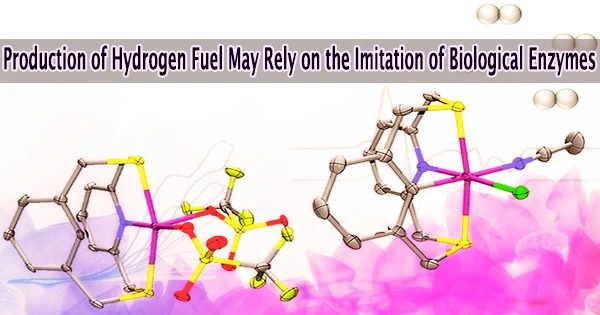
According to experts, an ancient biological enzyme called nickel-iron hydrogenase could be crucial in the production of hydrogen for an energy system based on renewable sources. Chemists at the University of Illinois Urbana-Champaign have created a synthetic molecule that mimics the chemical mechanism that the enzyme uses to produce hydrogen gas after careful research of the enzyme.
The researchers reported their findings in the journal Nature Communications.
Currently, industrial hydrogen is typically created by electrolyzing water to separate hydrogen gas molecules from oxygen atoms. Platinum metal is employed as a catalyst in the cathodes that drive this chemical process in the industrial setting to speed it up.
The cost and scarcity of platinum, however, make it undesirable as the globe moves toward more ecologically friendly energy sources, according to numerous studies.
On the other hand, nature’s nickel-iron hydrogenase enzyme produces hydrogen using earth-abundant metals in its core, said chemistry professor Liviu Mirica, who led the study with graduate student Sagnik Chakrabarti.
“The nickel at the core of the natural enzyme produces hydrogen by reducing protons in water,” Chakrabarti said. “During the catalytic process, the nickel center goes through paramagnetic intermediates, meaning that the intermediates have an unpaired electron which makes them extremely short-lived.”
“Synthetic chemists have made nickel compounds that produce hydrogen for over a decade,” Mirica said. While some of these compounds are highly effective hydrogen producers, the great majority of them work with non-paramagnetic intermediates.
One of the key takeaways from our work is that by using the specially designed ligand in the manner we did, we have successfully united ideas from two fields of inorganic chemistry bioinorganic and organometallic chemistry to make nickel complexes that behave similarly to the active site of one of nature’s most beautiful and complicated enzymes.
Sagnik Chakrabarti
“Researchers are trying to mimic exactly what nature does because it is efficient, and maximizing efficiency is a key challenge to overcome when engineering energy sources,” Mirica said. “Being able to reproduce the paramagnetic intermediate steps that occur in the natural enzyme is what our group is trying to achieve to increase efficiency and mimic nature.”
To accomplish this, the scientists created an organic molecule known as a ligand, which can hold the nickel in place and support the two crucial paramagnetic states that produce hydrogen. The ligand comprises electron-donating atoms like nitrogen and sulfur.
The existence of a carbon-hydrogen bond close to the nickel center, which is broken and reformed during catalysis, is the primary design feature that distinguishes this molecule from other catalysts. This was crucial in stabilizing the aforementioned paramagnetic states.
“One of the key takeaways from our work is that by using the specially designed ligand in the manner we did, we have successfully united ideas from two fields of inorganic chemistry bioinorganic and organometallic chemistry to make nickel complexes that behave similarly to the active site of one of nature’s most beautiful and complicated enzymes,” Chakrabarti said.
The researchers noted that other rare enzymes with metal-carbon linkages in their active regions have recently been discovered. Further understanding of how nature accomplishes chemistry with small molecules like hydrogen may be possible with the help of such design principles in synthetic complexes.
Former Illinois researchers Soumalya Sinha, Giang N. Tran and Hanah Na contributed to this study. The National Science Foundation supported this research.
Mirica also is affiliated with the neuroscience program, the Beckman Institute for Advanced Science and Technology and the Carle Illinois College of Medicine at Illinois.