Nickel succinate is a carboxylic acid salt of a transition metal. It crystallizes in a variety of forms. Nickel coordinates much more diversely than other transition elements, allowing for a wide range of structures for the same constituents. Because succinate is dibasic, its two ends can attach to two different nickel atoms. Because succinate is flexible, it can be bent to various angles and lengths. This allows metal-organic framework solids to form.
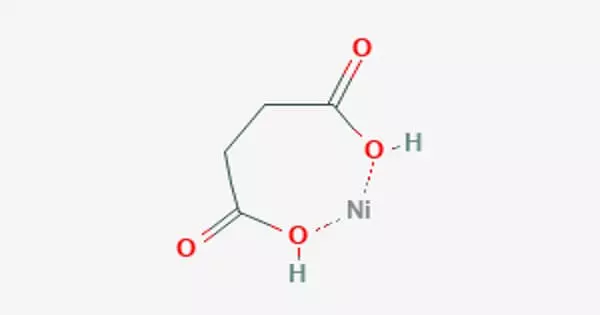
A neutral complex can be formed in a water solution by connecting one Ni2+ ion to both ends of a succinate ion.
Succinic acid and nickel carbonate can be used to produce the neutral nickel succinate NiC4H2O4•4H2O. Anhydrous nickel succinate dehydrates between 114 and 200°, Andydrous nickel succinate degrades at 380°, and over 450°C, only nickel oxide remains. Water, methane, ethylene, propane, and carbon dioxide are the most common gaseous decomposition products. Butene and pentane were minor byproducts. Two strong infrared absorption bands are present in the salt. One is at 3220-2950 cm-1 due to OH, and the other is at 1550 due to carboxylate –COO-.
Nickel is another metal ion from the first transition series that has been chosen for the synthesis and characterization of its complexes with the title dihydrazone. Its selection is based on the fact that a nickel or cobalt-promoted molybdenum catalyst is important in industrial catalysis, particularly in the hydrodesulfurization process, which involves the heterogeneous desulfurization of organosulfur compounds in petroleum feedstocks with dihydrogen.
Nickel is a silvery-white metal that occurs in cubic crystals. It is malleable and ductile, with high strength and corrosion resistance. The metal conducts heat and electricity well and has magnetic properties below 345°C. Nickel has five known isotopes. Nickel is chemically inert in its metallic form. It is insoluble in cold and hot water, ammonia, and concentrated nitric acid, and alkalis. However, it is soluble in dilute nitric acid and only slightly soluble in dilute hydrochloric and sulfuric acids.
Basic salts
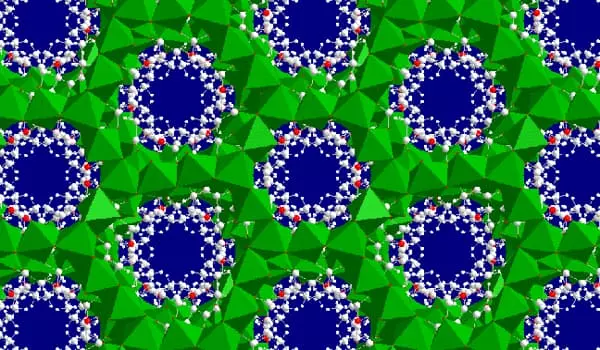
One structure called MIL-73, with the formula [Ni7(C4H2O4)4(OH)6·3H2O]·7H2O, crystallizes in the monoclinic system with a=7.8597 b=18.8154 c=23.4377 β=92.029° unit cell volume V=3463.9 Å3. The structure consists of alternating layers of nickel hydroxide chains running in the b direction stacked side by side in the direction, and succinate aligned in the c direction. Thermal decomposition resulted in stages of water loss at 45°, 60°, 110° (reversibly losing the ·7H2O. More water is lost at 225° and total decomposition occurs at 280°C.
Under 20K MIL-73 is ferrimagnetic.
Another structure contains a formula. [Ni7(C4H2O4)6(OH)2·3H2O]·7H2O. It has a honeycomb structure in three dimensions with pores running through it. [Ni7(C4H2O4)4(OH)2·2H2O]·2H2O is created by dissolving succinic acid in cyclohexanol and then mixing it with a nickel salt in water. This structure is extremely stable. It loses water between 200 and 240°C but maintains its structure until 380°C.