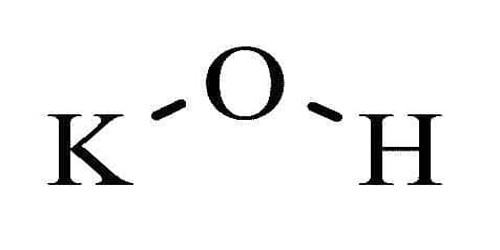Potassium hydroxide is a chemical compound. Its chemical formula is KOH. It contains potassium and hydroxide ions. It is an inorganic compound and is commonly called caustic potash. It is a strong alkali used in lake pigments, emulsions, and soaps with wax and resin, such as rosinate for varnishes.
It releases heat when it is dissolved in water, similar to sodium hydroxide. It gradually reacts with carbon dioxide in the air to form potassium carbonate. It melts easily. It feels greasy when it gets on the skin because it makes soap from the fat in your skin. It is made when potassium reacts with water.
Properties
It is a white powder. It dissolves easily in water to make a basic solution. It is corrosive to skin. It reacts with acids to give potassium salts. It is less common than sodium hydroxide. About 700,000 tons are made each year. It is hygroscopic, meaning that it absorbs water from the air and forms a solution. It is very hard to get the water out again.
- Chemical formula: [KOH]
- Molar mass 56.11 g mol−1
- Appearance: white solid, deliquescent
- Odor: odorless
- Density 2.044 g/cm3 (20 °C); 2.12 g/cm3 (25 °C)
- Melting point 360°C (680 °F; 633 K); Boiling point 1,327 °C (2,421 °F; 1,600 K)
- Solubility in water: 85 g/100 mL (-23.2 °C); 97 g/100 mL (0 °C); 121 g/100 mL (25 °C); 138.3 g/100 mL (50 °C); 162.9 g/100 mL (100 °C)
- Solubility: soluble in alcohol, glycerol insoluble in ether, liquid ammonia
- Solubility in methanol 55 g/100 g (28 °C)
Preparation
It is made by electrolysis of a potassium chloride solution. It can also be made by reacting calcium hydroxide with potassium carbonate. The calcium carbonate precipitates and is filtered, leaving potassium hydroxide.
Uses
Potassium hydroxide is an inorganic compound used in a range of industrial applications. It is used in various chemical, industrial and manufacturing applications. It is used to make soap. It is also used in organic chemistry. It can be used to make fertilizers and other potassium compounds. It is used in food to adjust pH, as a stabilizer, and as a thickening agent.It is used to make diesel from plants. It is used in alkaline cells and nicads as an electrolyte. It can be used to identify mushrooms. KOH can be used interchangeably for a number of applications, although in industry, NaOH is preferred because of its lower cost.
Safety
Potassium hydroxide and its solutions are severe irritants to the skin and other tissue.
Information Source: