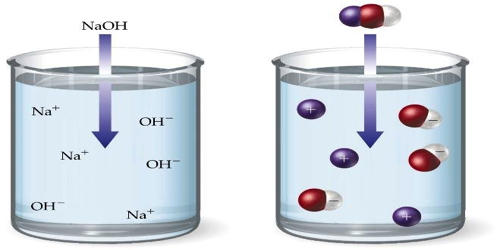
Aqueous is a term used to define a system that involves water. An aqueous solution is a solution in which the solvent is water. It is the combination of one or more items in which water is the solvent that the solute dissolves in. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be represented as Na+(aq) + Cl−(aq). The word aqueous (which comes from aqua) means pertaining to, related to, similar to, or dissolved in water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. However, water is only a liquid at room temperature, as a liquid is the state of an item. Ice is water too, but it is in a solid-state when frozen. An aqueous solution is a water with a pH of 7.0 where the hydrogen ions (H+) and hydroxide ions (OH−) are in the Arrhenius balance (10−7).
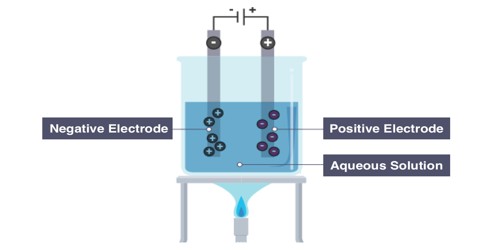
A non-aqueous solution is a solution in which the solvent is a liquid, but is not water. It often allows for conducting electricity. Solutions that contain strong electrolytes tend to be very good electrical conductors such as seawater. Substances that are hydrophobic (‘water-fearing’) often do not dissolve well in water, whereas those that are hydrophilic (‘water-friendly’) do. An example of a hydrophilic substance is sodium chloride. Acids and bases are aqueous solutions, as part of their Arrhenius definitions. In such reactions, the cation from one reactant takes the place for the cation in the other reactant. Hence it is typically forming an ionic bond.
Three different types of reactions with aqueous solutions are as follows:
(1) Precipitation Reactions
These reactions take place when two aqueous reactants, one solid and one liquid, react to form an insoluble product which is called a precipitate. For example when lead nitrate mixes with potassium iodide as shown in the following chemical reaction:
Pb(NO3)2 + 2KI ⇒ PbI2 + 2KNO3
Lead iodide which produces here is not soluble product and hence is the precipitate.
(2) Acid-base Reaction
As we know acid contains positive hydrogen ions. On the other hand, a base is a substance that accepts hydrogen ions and produces negative hydroxyl ions in water. For example, a neutralization reaction occurs when HCl acid combines with NaOH to produce water and sodium chloride. The chemical equation is:
HCl + NaOH ⇒ H2O + NaCl
(3) Oxidation-Reduction Reactions
Oxidation is the process in which a chemical loses electrons and hence becomes more positive. Whereas Reduction is the opposite process in which a chemical gains electrons and hence becomes more negative. An oxidation-reduction reaction takes place between a metal and a non-metal. For example when sodium reacts with chlorine and they produce sodium chloride:
2Na + Cl2 ⇒ 2NaCl
Reactions in aqueous solutions are usually metathesis reactions. Metathesis reactions are another term for double-displacement; that is when a cation displaces to form an ionic bond with the other anion. The cation bonded with the latter anion will dissociate and bond with the other anion.