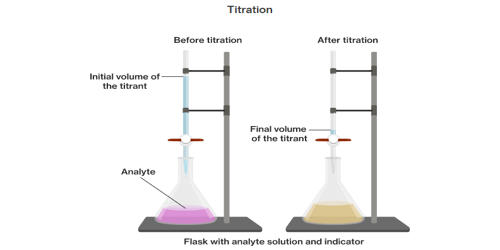
The analyte is a substance or chemical constituent that is of interest in an analytical procedure. An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. It is a substance whose chemical constituents are being identified and measured. It is a chemical substance that is the subject of chemical analysis. The substance that you are trying to find the concentration by using the standard solution.
The analyte is prepared by dissolving the substance being studied into a solution. The solution is usually placed in a flask for titration. The purest substances are referred to as analytes. Example: 24 karat gold, NaCl, water, etc. In reality, no substance has been found to be 100% pure in its quality, so we call a substance that is found to be most pure (for some metals, 99% after electrolysis) an analyte. The standard solution is usually added to the solution containing the analyte by means of a buret, a piece of volumetric glassware capable of accurately measuring solution volumes. It is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte
An analyte is any chemical species involved in the reaction of the titration eg., Sodium ions. The target analyte is the unknown analyte which you are trying to identify the concentration of. In laboratory and layman jargon the “property” is often left out provided the omission does not lead to an ambiguity of what property is measured. The technique known as titration is an analytical method commonly used in chemistry laboratories for determining the quantity or concentration of a substance in a solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the “analyte”) until the equivalence point is reached. To perform titration, the conventional way involves using a burette to dispense a measured volume of liquid. It is a substance or sample being analyzed, usually by means of a laboratory procedure or test. Since volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the titrant or titrator, is prepared as a standard solution. The graduated equipment in the laboratory used to dispense the titrant which reacts with the solution to form an observable chemical change is called a burette. Detection of low analyte concentrations in complex biological, pharmaceutical, or environmental samples continues to be a challenge.