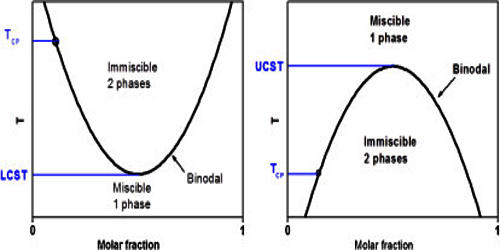
Critical solution temperature is the temperature at which complete miscibility is reached as the temperature is raised or in some cases lowered —used of two liquids that are partially miscible under ordinary conditions. The upper critical solution temperature (UCST) is the critical temperature above which the components of a mixture are miscible in all proportions. It is the temperature, below which ferrite starts to form as a result of ejection from austenite in the hypereutectoid alloys. The word upper indicates that the UCST is an upper bound to a temperature range of partial miscibility or miscibility for certain compositions only. For example, hexane-nitrobenzene mixtures have a UCST of 19°C, so that these two substances are miscible in all proportions above 19°C but not at lower temperatures. Examples at higher temperatures are the aniline-water system at 168°C (at pressures high enough for liquid water to exist at that temperature), and the lead-zinc system at 798°C (a temperature where both metals are liquid).
A solid-state example is a palladium-hydrogen system which has a solid solution phase (H2 in Pd) in equilibrium with a hydride phase (PdHn) below the UCST at 300°C. Above this temperature, there is a single solid solution phase. It is the temperature, below which cementite starts to form as a result of ejection from austenite in the hypereutectoid alloys.
The UCST depends upon the pressure and molar-mass distributions of the constituent polymer(s). In the phase diagram of the mixture components, the UCST is the shared maximum of the concave down spinodal and binodal (or coexistence) curves. The UCST is in general dependent on pressure. For a mixture containing or consisting of polymeric components, these may be different polymers or species of the different molar mass of the same polymer.
Polymers showing an upper critical solution temperature (UCST) in aqueous solution were not rare, but the UCST was rarely observed under practically relevant conditions. The phase separation at the UCST is in general driven by unfavorable energetics; in particular, interactions between components favor a partially demixed state. Current developments focus on polymers that rely on hydrogen bonding.