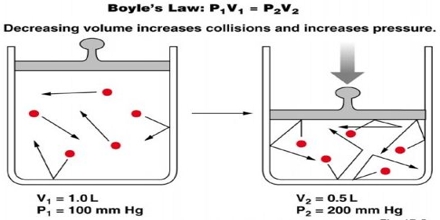
Major objective of this lecture is to explain on Boyle’s Law. Law States that: ‘for a fixed mass of gas kept at constant temperature the volume of the gas is inversely proportional to its pressure’. Boyle’s Law is one of the laws in physics that concern the behaviour of gases; when a gas is under pressure it takes up less space: the higher the pressure, the smaller the volume. This law tells us about the relationship between the volume of a gas and its pressure at a constant temperature.
The law states that pressure is inversely proportional to the volume.