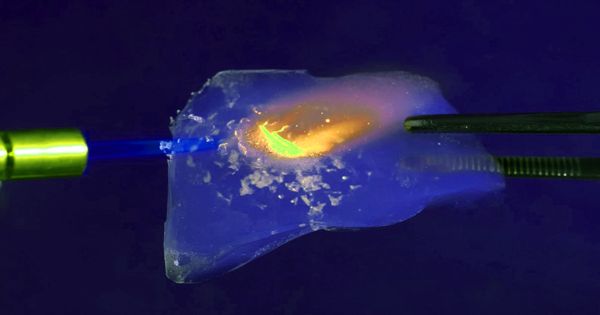
Aerogels are among the lightest solid materials known to man. Aerogel is a synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with gas without significant collapse of the gel structure. They are created by combining a polymer with a solvent to form a gel, and then removing the liquid from the gel and replacing it with air. The result is a solid with extremely low density and extremely low thermal conductivity. It is not a specific mineral or material with a set chemical formula-rather, the term is used to encompass all materials with a specific geometrical structure.
Aerogels are a class of highly porous materials from almost any kind of composition, namely metals, semiconductors, oxides, polymers, biopolymers, hybrids, and carbon, to name only a few.
Aerogels are extremely porous and very low in density. Nicknames include frozen smoke, solid smoke, solid air, solid cloud, blue smoke owing to its translucent nature and the way light scatters in the material. They are solid to the touch. Silica aerogels feel like fragile expanded polystyrene to the touch, while some polymer-based aerogels feel like rigid foams. This translucent material is considered one of the finest insulation materials available. Aerogels can be made from a variety of chemical compounds. This structure is an extremely porous, solid foam, with high connectivity between branched structures of a few nanometres across.
Aerogel was first created by Samuel Stephens Kistler in 1931, as a result of a bet with Charles Learned over who could replace the liquid in “jellies” with gas without causing shrinkage. Since their invention, aerogels have primarily been made of silica. The silica is combined with a solvent to create a gel. One of the best known and most useful physical properties of aerogel is its incredible lightness-it typically has a density between 0.0011 to 0.5 g cm-3, with a typical average of around 0.020 g cm-3.
Aerogels are produced by extracting the liquid component of a gel through supercritical drying or freeze-drying. This gel is then subjected to supercritical fluid extraction. This allows the liquid to be slowly dried off without causing the solid matrix in the gel to collapse from capillary action, as would happen with conventional evaporation. This supercritical fluid extraction involves introducing liquid carbon dioxide into the gel. The carbon dioxide surpasses its supercritical point, where it can be either a gas or a liquid, and then is vented out.
The first innovation is a method of creating aerogels that are reinforced by polymers. The first aerogels were produced from silica gels. The method changes the surface of the gel as it reacts with a polymer. Aerogels provide very effective insulation because they are extremely porous and the pores are in the nanometer range. Kistler’s later work involved aerogels based on alumina, chromium, and tin dioxide. The nanopores aren’t visible to the human eye. Carbon aerogels were first developed in the late 1980s.
Information Source: