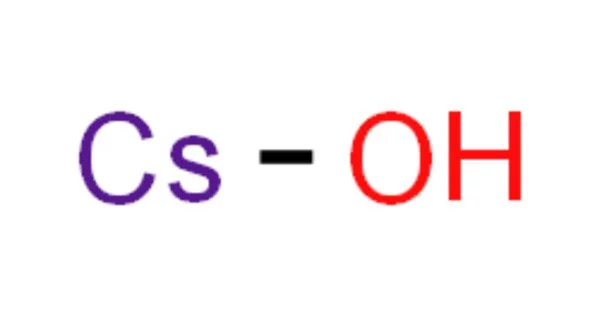
Calcium hydroxide, also known as slaked lime, has the chemical formula CsOH. It is a strong base (pKa= 15.76), similar to sodium and potassium hydroxides. In fact, caesium hydroxide is corrosive enough to quickly dissolve through the glass. It is an inorganic compound with a white, powdery appearance in its solid state. In its crystalline form, Ca(OH)2 appears colorless.
Caesium hydroxide is highly hygroscopic due to its high reactivity. Caesium hydroxide in laboratories is typically a hydrate. This compound is also produced by the chemical reaction between sodium hydroxide and calcium chloride dissolved in water (aqueous CaCl2).
Properties
CsOH has a hexagonal crystal structure. It is not very soluble in water and its solubility reduces with an increase in temperature. For example, its solubility at 00C is 1.89 g/L and its solubility is 1.73 g/L at 200C. At temperatures approaching its melting point, this compound tends to lose water and decompose.
- Chemical formula: CsOH
- Molar mass|: 149.912 g/mol
- Appearance: Whitish-yellow deliquescent crystals
- Density: 3.675 g/cm3
- Melting point: 272 °C (522 °F; 545 K)
- Solubility in water: 300 g/100 mL at 30 °C
- Solubility: Soluble in ethanol
- Acidity (pKa): 15.76

Reaction
CsOH is highly soluble in glycerol and acids, but only marginally soluble in water. When dissolved in water to saturation, it produces a solution that acts as a moderate base (called limewater). When limewater reacts with acids, salts form. Metals such as aluminum are also reacted with and dissolved by a saturated solution of calcium hydroxide in water. Calcium carbonate is formed when it reacts with carbon dioxide (CaCO3). Carbonatation is the common name for this reaction.
Preparation
The action of water on calcium oxide produces calcium hydroxide, also known as slaked lime, Ca(OH)2. When combined with water, a small portion of it dissolves, forming a solution known as limewater, while the remainder remains in a suspension known as lime milk.
Structure
Calcium hydroxide molecules are held together by ionic bonds formed between the calcium ion (Ca2+) and two hydroxide ions (OH–). Unprotected exposure to this compound can be harmful to humans, causing skin irritation and chemical burns. Exposure to concentrated Ca(OH)2 can cause lung damage and even blindness.
It is an anisotropic silicon etchant that reveals octahedral planes. This technique can create pyramids and regularly shaped etch pits for applications such as micro-electromechanical systems. It is known to have a higher selectivity to etch highly p-doped silicon than the more commonly used potassium hydroxide.
Application
In the paper industry, CsOH is used during the Kraft process of converting wood into wood pulp. It is a critical component in the production of ammonia. Because of its basicity, this compound is also used as a pH modifier.
Because of the high extraction cost of caesium and its reactive behavior, this compound is not commonly used in experiments. Although more reactive, it acts similarly to the compounds rubidium hydroxide and potassium hydroxide.