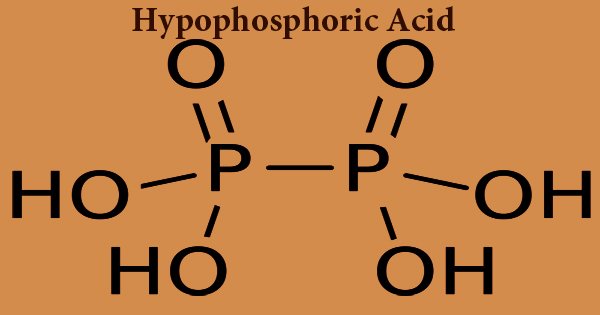
Hypophosphoric acid is a mineral acid with the formula H4P2O6, also known as hypophosphite, with phosphorus in a formal oxidation state of +4. It is a colorless oil or crystal of deliquescence, a significant fine chemical substance. In addition, it exists as the dihydrate, H4P2O6·2H2O in the solid-state. H3PO2 is usually written as the formula for hypophosphorous acid, but HOP(O)H2 is a more descriptive presentation that emphasizes its monoprotic character. Phosphinates are called salts derived from this acid (hypophosphites). Isohypophosphoric acid is a hypophosphoric acid structural isomer in which one phosphorus has a hydrogen directly bound to it and that phosphorus atom is linked to the other by an oxygen bridge to provide a mixed anhydride of phosphorous acid/phosphoric acid. The two phosphorus atoms are in the +3 and +5 oxidation states, respectively.
Hypophosphoric acid can be produced by reacting at room temperature with red phosphorus and sodium chlorite. It can also be used in the esterification reaction catalyst, the refrigerant, in particular for the development of high purity sodium hypophosphite product, as a reducing agent for electroless plating, phosphoric prevention of discoloration of resins. Hypophosphoric acid consists of oxonium ions and is best formulated (H3O+)2 (H2P2O6)2−. The (HOPO2PO2OH)2- anion with a P-P bond length of 219 pm is isostructural with diammonium salt.
Hypophosphoric acid can be prepared at room temperature through the reaction of red phosphorus with sodium chlorite.
2 P + 2 NaClO2 + 2 H2O → Na2H2P2O6 + 2 HCl
The popular industrial method of production is the method of ion exchange resin and the method of electrodialysis. There are many preparation methods. If white phosphorus oxidizes in the air when partially submerged in water, a mixture of hypophosphoric acid, phosphoric acid (H3PO3), and phosphoric acid (H3PO4) is generated. They are crystals that are deliquescent or colorless resin. Point of melting: 26.5°C; Relative (specific gravity) density: 1.439 (solid, 19°C). It is soluble in water, ethanol, and ether and can be blended with water, ethanol, and acetone in all proportions. It deliquesces quickly into syrupy liquid in the air, and the aqueous solution is acidic.
The asymmetric, staggered structure of the HOPO2PO2OH2- anion in Na2H2P2O6·6H2O is similar to that of ethane. In addition, it has a length of 219 pm P−P bond. There are two P-O bonds with a length of 151 pm and a P-OH bond length of 159 pm for each phosphorus atom. The Na4P2O6·10H2O tetrasodium salt crystallises at pH 10 and the Na2H2PO6·6H2O disodium salt crystallises at pH 5.2. To form the acid dihydrate, H4P2O6·2H2O, the disodium salt can be transferred through an ion-exchange column.
Under reduced pressure and low-temperature crystallization, hypophosphorous acid can be obtained by evaporating. The solubility of the barium salt is small because of this operation, so the concentration of hypophosphorous acid obtained is not high, and the industrial product should be cleaned by recrystallization. Hypophosphoric acid is unstable in hot hydrochloric acid, in 4 M HCl it hydrolyses to give H3PO3 + H3PO4.
Hypophosphoric acid can be prepared by reacting at room temperature with red phosphorus and sodium chlorite.
2 P + 2 NaClO2 + 2 H2O → Na2H2P2O6 + 2 HCl
It oxidizes in the air when white phosphorus is partially dissolved in water, resulting in a mixture of hypophosphoric acid, phosphoric acid, and phosphoric acid. Hypophosphates tend to oxidize into pyrophosphates containing the P2O4-7 ion while they are in the air, where P has a formal oxidation state of +5. Hypophosphates in alkali hydroxides are stable. They rapidly transform to the orthophosphate containing PO3−4 infused sodium hydroxide.
Hypophosphorous acid is used as a reducing agent for electroless plating; it may be used to prevent phosphoric acid resin from decoloring; it is used as a refrigerant esterification catalyst. It is used as a nourishing agent for the processing of hypophosphite, sodium salts, manganese salts, and iron salts. In medicine and as a reducing agent, hypophosphorous acid is used for the determination of arsenic, tellurium, and the isolation of tantalum, niobium, and other reagents.
Hypophosphoric acid is a liquid that is corrosive. Chemical burns to the skin and eyes may result in it. In addition, it can cause inflammation in the upper respiratory tract if someone inhales it. Not only that, it can cause eye and eyelid irritation. It is recommended that water should always be used to extract it.
Information Sources: