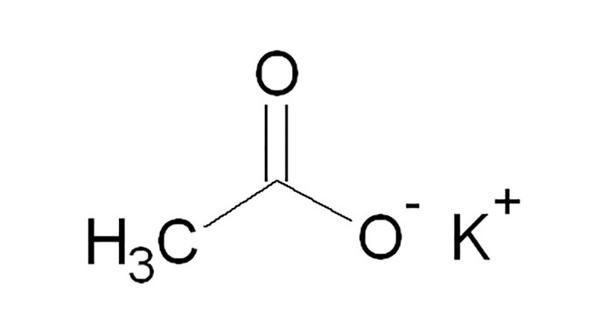
Potassium Acetate is the acetate salt form of potassium, an essential macromineral. Its chemical formula is CH3COOK. It is the potassium salt of acetic acid. It is also called Diuretic salt or Potassium ethanoate, or Acetic acid potassium salt. It is a hygroscopic solid under room temperature. It is an essential macro-mineral and a potassium salt which consists of an equal number of acetate and potassium ions.
Potassium maintains intracellular tonicity, is required for nerve conduction, cardiac, skeletal and smooth muscle contraction, production of energy, the synthesis of nucleic acids, maintenance of blood pressure and normal renal function. It helps in maintaining intracellular tonicity, which is required for nerve conduction, smooth muscle contraction, normal renal function, and maintenance of blood pressure.
Properties
Diuretic salt is a deliquescent white crystalline powder. It is soluble in water and has a pH value between 7.5-9.0.
- Molecular weight: 98.142 g/mol
- Appearance: White Powder
- Density: 1.8 g/cm3
- Boiling point: Decomposes
- Melting Point: 292 °C
- Exact Mass: 97.977 g/mol
- Monoisotopic Mass: 97.977 Da.

Preparation
It can be prepared by treating a potassium-containing base such as potassium hydroxide or potassium carbonate with acetic acid:
CH3COOH + KOH → CH3COOK + H2O
This sort of reaction is known as an acid-base neutralization reaction. At around temperature range of 41.3°C the sesquihydrate in water solution starts forming semi hydrates.
Applications – It is widely used to replenish electrolytes, as a urinary and systemic alkalizer. Earlier it was used in expectorants and diuretics.
- Deicing – Potassium acetate can be used as a deicer to remove ice and prevent its formation. It is preferred for airport runways although it is more expensive.
- Fire extinguishing – It is the extinguishing agent used in Class K fire extinguishers because of its ability to cool and form a crust over burning oils.
- Food additive – It is used in processed foods as a preservative and acidity regulator.
- Medicine and biochemistry – In medicine, potassium acetate is used as part of electrolyte replacement protocols in the treatment of diabetic ketoacidosis because of its ability to break down to bicarbonate to help neutralize the acidotic state. In molecular biology, potassium acetate is used to precipitate dodecyl sulfate (DS) and DS-bound proteins to extract ethanol from DNA.
- Industry – It is used as a catalyst in the production of polyurethanes.
Information Source: