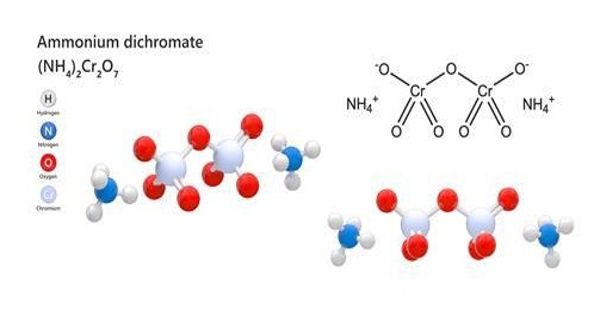
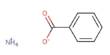
Ammonium dichromate is a flammable inorganic compound. It is an inorganic compound with the formula (NH4)2Cr2O7. It is orange-red needles produced during crystallization. In this compound, as in all chromates and dichromates, chromium is in a +6 oxidation state, commonly known as hexavalent chromium. It is a toxic chemical salt used to sensitize organic emulsions such ad gelatin to the hardening effect of actinic light. It is a salt consisting of ammonium ions and dichromate ions. It is more sensitive to light.
Ammonium dichromate is a bright orange-red crystalline solid. It may act as a strong oxidizing agent if mixed with or contaminated with combustible material.
Ammonium dichromate is sometimes known as Vesuvian Fire, because of its use in demonstrations of tabletop “volcanoes”. Upon heating, ammonium dichromate decomposes to create nitrogen dioxide, water vapor (Water is gaseous in this reaction due to the relatively high reaction temperature), and solid chromium (III) oxide. However, this demonstration has become unpopular in schools due to the compound’s carcinogenic nature. It has also been used in pyrotechnics and in the early days of photography.

Properties
At room temperature and pressure, the compound exists as orange, acidic crystals soluble in water and alcohol. It is readily ignited and burns to produce a voluminous green residue. It is formed by the action of chromic acid on ammonium hydroxide with subsequent crystallization.
- Density: 2.12 g/cm³
- Molecular Weight/ Molar Mass: 252.07 g/mol
- Autoignition temperature: 190 °C
- Melting point: 180 °C
- Appearance: Orange-red crystals
- Stability: Stable under recommended storage conditions
- Specific Gravity: 2.1500
- Solubility: Insoluble in acetone; soluble in alcohol
The ammonium dichromate molecule can be considered an ionic compound because it features an ionic bond between the ammonium cation and the dichromate anion. The (NH4)2Cr2O7 crystal (C2/c, z=4) contains a single type of ammonium ion, at sites of symmetry C1(2,3). Each NH4+ center is surrounded irregularly by eight oxygen atoms at N—O distances ranging from ca. 2.83 to ca. 3.17 Å, typical of hydrogen bonds. However, the individual polyatomic cation and anion are held together by covalent bonds.
Uses
- Ammonium dichromate is used in sensitizing solutions used in lithography.
- It has been used in pyrotechnics and in the early days of photography as well as in lithography, as a source of pure nitrogen in the laboratory, and as a catalyst.
- It is used in pyrotechnics, lithography, and photoengraving. It is also used as a magnetic recording material.
- It is also used as a mordant for dyeing pigments, in the manufacturing of alizarin, chrome alum, leather tanning, and oil purification. It is used as an approved pesticide and used as a mordant for dyeing, used as a pigment.
Information Source: