An acid-base reaction is a chemical reaction that occurs between an acid and a base. It is a type of chemical process typified by the exchange of one or more hydrogen ions, H+, between species that may be neutral or electrically charged. It can be used to determine pH. The reaction between an acid and a base is known as a neutralization reaction. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid-base theories, for example, Brønsted–Lowry acid-base theory. There are some factors to consider when looking at an acid-base reaction. The strength of the acid or base is very important in determining the overall process that is occurring. A strong acid or a strong base will ionize completely, where a weak acid or weak base will only ionize partially.
Example
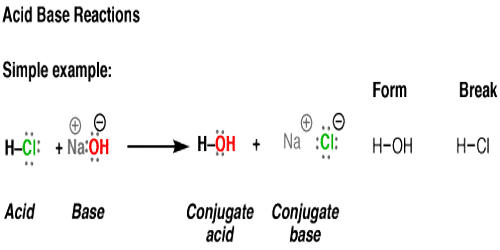
The reaction between a strong acid and a strong base will always result in the formation of water and an ionic salt (remember that all ionic substances are salts in their solid form, not just NaCl). The reaction between a strong acid and a strong base is written as follows: Acid + Base à water + salt for strong acid + strong base. An example is shown below:
(1) Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (a salt) and water. Sodium chloride is made up of Na+Na+ cations from the base (NaOH)(NaOH) and Cl−Cl− anions from the acid (HCl)(HCl).
HCl (aq)+NaOH (aq)→H2O (l)+NaCl (aq)HCl (aq)+NaOH (aq)→H2O (l)+NaCl (aq)
(2) Hydrochloric acid reacts with ammonia to form ammonium chloride (a salt). Ammonium chloride is made up of NH+4NH4+ cations from the base (NH3)(NH3) and Cl−Cl− anions from the acid (HCl)(HCl).
HCl (aq)+NH3(aq)→NH4Cl (aq)
When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt. The H(+) cation of the acid combines with the OH(-) anion of the base to form water. An example of a weak basic solution is seawater, which has a pH near 8.0, close enough to neutral that well-adapted marine organisms thrive in this alkaline environment.
Their importance becomes apparent in analyzing acid-base reactions for gaseous or liquid species, or when acid or base character may be somewhat less apparent. The idea that some substances are acids whereas others are bases is almost as old as chemistry, and the terms acid, base, and salt occur very early in the writings of the medieval alchemists. The first of these concepts was provided by the French chemist Antoine Lavoisier, around 1776.
One of the first things that were noted about acids is that they have a sour taste. It is important to think of the acid-base reaction models as theories that complement each other. For example, the current Lewis model has the broadest definition of what an acid and base are, with the Bronsted-Lowry theory being a subset of what acids and bases are, and the Arrhenius theory being the most restrictive. Bases were noted to have a soapy feel and a bitter taste. However, you cannot go around tasting and feeling unknown substances since they may be harmful.