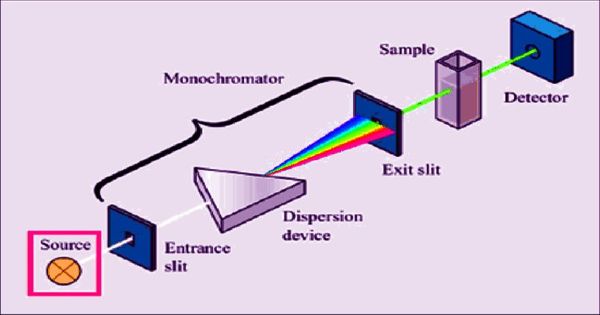
Absorbance spectroscopy is a molecular spectroscopy method that uses the wavelength-dependent absorption characteristics of materials to identify and quantify specific substances. It is a technique used to find out what makes up a sample of a substance – in other words, a chemical analysis. The absorbance of a solution increases as the attenuation of the optical beam increases. Absorption spectroscopy measures how much light is absorbed by a sample over a range of wavelengths defined by the electromagnetic spectra
When a full spectrum of light (light with all the colors, like light from the sun) passes through the sample (which is often a gas) some specific colors do not show up on the other side. Infrared and ultraviolet-visible spectroscopy is particularly common in these kinds of analytical applications. These colors of light are being absorbed by the sample. It works as an analytical chemistry tool that can determine if a particular substance is present in a sample and often also quantify how much of the substance is present.
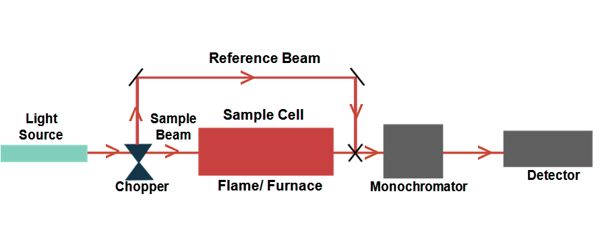
There are many different approaches for measuring absorption spectra. An image is created of the spectrum of light with black breaks where the light has been absorbed. These breaks are called absorption lines, and every element has its characteristic pattern of absorption lines. The most common one is to point a generated beam of light at a sample and detect the intensity of the radiation that goes through it.
The absorption of light by atoms provides a powerful analytical tool for both quantitative and qualitative analysis. On an atomic scale, this happens because of the electrons in the atoms of the sample – an electron can absorb light to gain energy. Atomic absorption spectroscopy (AAS) is based upon the principle that free atoms in the ground state can absorb light of a certain wavelength. From experiments, electrons only ever absorb certain amounts of energy, suggesting an electron’s energy must fit onto the set, quantized, discrete energy levels. Absorption for each element is specific, no other elements absorb this wavelength.
There is a wide range of experimental approaches for measuring absorption spectra. The process of an electron going to a higher energy level is called excitation. The most common arrangement is to direct a generated beam of radiation at a sample and detect the intensity of the radiation that passes through it. For any atom of a particular element, the energy needed to excite an electron from one specific energy level to another will be the same.
Information Source: