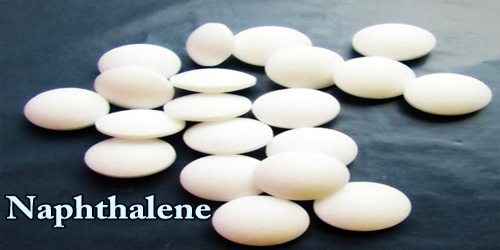
Naphthalene is a white, volatile, solid polycyclic hydrocarbon with a powerful smell of mothball, and its chemical formula is C10H8. It is detectable at concentrations as low as 0.08 ppm by mass. It is an important raw material of hydrocarbon which gives rise to a host of substitute products used in the manufacture of synthetic resins and dyes.
Naphthalene is obtained from either pitch or petroleum distillation and is primarily wont to manufacture anhydride, but is additionally employed in moth repellents. It the foremost abundant single constituent of the pitch, a volatile product from the fractional process of coal, and is additionally formed in modern processes for the high-temperature cracking (breaking of large molecules) of petroleum. As a hydrocarbon, naphthalene’s structure consists of a fused pair of benzene rings. It’s best referred to as the most ingredient of traditional mothballs.
Naphthalene is manufactured commercially by crystallizing from the intermediate fraction of condensed coal tar and the heavier fraction of cracked petroleum. The substance crystallizes in lustrous white plates, melting at 80.1° C (176.2° F) and boiling at 218° C (424° F). Naphthalene exposure is associated with hemolytic anemia, hepatic and neurological damage, cataracts, and retinal hemorrhage.
Naphthalene is nearly insoluble in water; it’s highly volatile and incorporates a characteristic odor. It’s been used as a moth repellent. Naphthalene is fairly anticipated to be somebody’s carcinogen and will be related to an increased risk of developing laryngeal and colorectal cancer. Naphthalene’s chemical formula was determined by Michael Faraday in 1826. The main industrial use of naphthalene is in the manufacture of plastics made from polyvinyl chloride (PVC). The primary use is in moth repellents and deodorant blocks for toilets.
Naphthalene displays the aromatic character associated with benzene and its pure derivatives in its chemical behavior. Unlike benzene, the molecule is planar; the carbon bonds in naphthalene are not of the same length as benzene. The bonds C1−C2, C3−C4, C5−C6, and C7−C8 are about 1.37 Å (137 pm) in length, whereas the other carbon bonds are about 1.42 Å (142 pm) long. Its reactions are mainly the substitution of hydrogen atoms by halogen atoms, nitro groups, sulfonic acid groups, and alkyl groups.
Naphthalenes solid is denser than water and insoluble in water; Burns, but could also be difficult to ignite. Within the molten form, it’s extremely popular. Exposure to skin must be avoided. Large quantities of naphthalene are converted to naphthylamines and naphthols to be used as dyestuff intermediates. For several years naphthalene was the principal material for creating anhydride.
Naphthalene can be hydrogenated under high pressure in the presence of metal catalysts to give 1,2,3,4-tetrahydronaphthalene (C10H12), also known as tetralin. In both cases, naphthalene was metabolized into a product(s) that was covalently bound and into two major methanol-soluble compounds co-chromatographed with 1-naphthol and 1,2-dihydro-1,2-dihydroxy naphthalene. Throughout the petroleum refining process, large amounts of naphthalene were also produced from heavy petroleum fractions, but today petroleum-derived naphthalene represents only a minor component of naphthalene. Naphthalene produces species and cell-selective injury to tract epithelial cells of rodents. Naphthalene and its alkyl homologs are the most important constituents of creosote. It’s employed in engineering to check heat transfer using mass sublimation.
Information Sources:



![Report on Beximco Knitting Ltd [ Part-2]](https://assignmentpoint.com/wp-content/uploads/2013/04/Beximco-Knitting-Limited-110x55.jpg)








