Sodium ethoxide is an organic monosodium salt which contains ethoxide as its counterpart; it’s chemical formula C2H5ONa. Even though impure samples appear yellow or brown, it is a white solid; it dissolves in polar solvents such as ethanol. Typically used as a solid foundation. It has a function as a nuclear reagent and contains an ethoxide. Owing to oxidation, solid samples of sodium ethoxide gradually turn dark when placed in dry air.
Sodium ethoxide may be a white or yellowish powder; hygroscopic; darkens and decomposes on exposure to air; decomposes in water forming hydroxide and ethanol; dissolves in absolute ethanol. It’s commercially available and as an answer in ethanol. It is easily prepared in the laboratory by treating sodium metal with absolute ethanol:
2 C2H5OH + 2 Na → 2 C2H5ONa + H2
Sodium ethoxide is primarily used for the condensation reactions in organic synthesis. In several organic reactions, it is likewise a catalyst. It is highly inflammable; on contact with water the possibility of fire and explosion. An alternative, the cheaper route involves the reaction of hydrated oxide with anhydrous ethanol. This reaction suffers from incomplete conversion to the alkoxide, except for less stringent applications, the full conversion is unimportant.
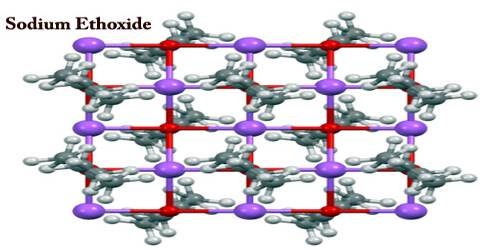
Sodium hydroxide is formed when sodium ethylate is exposed to moisture, as in caustic soda, ethanol. The sodium ethoxide crystal structure was determined with X-ray crystallography. It consists of layers of alternating Na+ and O− centers with disordered ethyl groups covering the top and bottom of each layer. At 10° C sodium is added to pure alcohol in limited amounts. The temperature rises to warm (about 38° C). The ethyl layers are packed back-to-back which results in a lamellar structure. It is a dangerous accumulation of airborne particles which can be easily reached when dispersed.
The reaction of sodium and ethanol sometimes forms other products such as the disolvate NaOEt·2EtOH. The crystal structure was determined while the structure of other phases remains uncertain in the Na / EtOH framework. Other impurities are NaOH and Na2CO3 if held in the air for long periods. In this case, the powder cannot be used if these impurities affect the reactivity, and a fresh sample should be acquired. Sodium ethoxide could be a strong base and is therefore corrosive. Take off dirty garments; Rinse with plenty of water or shower on skin. Sodium ethoxide can either deprotonate an ester molecule’s α-position, form an enolate or the ester molecule may undergo a nucleophilic substitution called transesterification.
Information Sources: