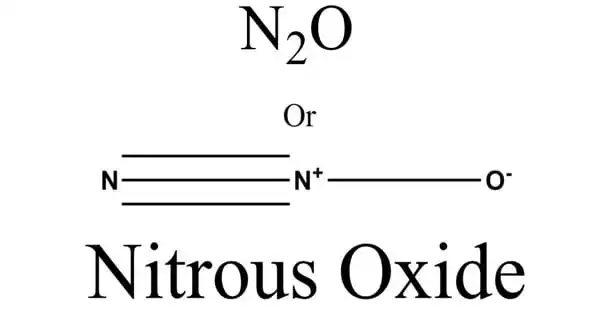
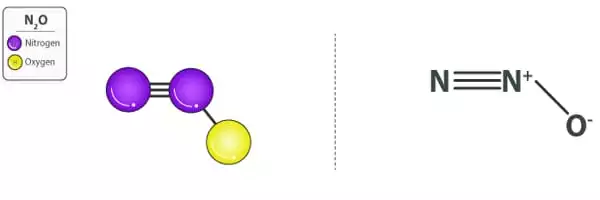
Nitrous oxide, also known as laughing gas or nitrous oxide, is a chemical substance having the formula N2O. It is a colorless gas with a pleasant, sweetish odor and taste that, when inhaled, causes insensibility to pain, followed by moderate hysteria and, occasionally, laughter. It is a colorless, non-flammable gas with a little metallic odor and taste at room temperature. Nitrous oxide, like molecular oxygen, is a potent oxidizer at high temperatures.
In 1772, the English chemist Joseph Priestley discovered nitrous oxide; another English chemist, Humphry Davy, later named it and demonstrated its physiological impact.
Properties
Nitrous oxide is a white gas with a pleasant taste. It is also referred to as “laughing gas.” Continued inhalation of the fumes may impair decision-making. It is noncombustible, however, it will hasten the combustion of combustible material in a fire. It dissolves in water. Its fumes weigh more than air.
- Molecular Weight/ Molar Mass: 44.013 g/mol
- Density: 1.98 kg/m³
- Boiling Point: – 88.48 °C
- Melting Point: − 90.86 °C
Preparation
Nitrous Oxide is a colorless, non-flammable gas that occurs naturally. It can be made and utilized as a pharmacologic agent to create anesthesia, a food addition as a propellant, and a fuel additive to boost accessible oxygen in combustion.
It is made by the action of zinc on dilute nitric acid, hydroxylamine hydrochloride (NH2OH•HCl) on sodium nitrite (NaNO2), and, most often, the decomposition of ammonium nitrate (NH4NO3).
Nitrous oxide is usually made from ammonia nitrate. Some consideration must be given to the cleanliness of the salt, which should contain no ammonia hydro chlorate. It is made by combining crushed ammonia carbonate with pure nitric acid, which has been previously diluted with half its weight of water as long as there is effervescence and a minor excess of the carbonate may be left in the liquor at the end. The solution is concentrated when its boiling point exceeds 250oC and a drop of it solidifies on a cool glass plate. To produce nitrous oxide, a portion of this salt is placed in a retort and heated with a charcoal choffer, the diffused heat of which is preferable to the heat of the lamp.
Nitrous oxide concentrations in the atmosphere reached 333 parts per billion (ppb) in 2020, increasing at a pace of roughly 1 ppb each year. It is a significant scavenger of stratospheric ozone, with an impact comparable to CFCs.
Applications
Nitrous oxide is mostly used as an anesthetic in short-term surgical procedures; prolonged inhalation results in death. In addition, the gas is employed as a propellant in food aerosols. Nitrous oxide is injected into an engine’s air intake in automobile racing; the increased oxygen allows the engine to burn more fuel each stroke.
Nitrous oxide has numerous medicinal applications, particularly in surgery and dentistry, due to its anesthetic and pain-relieving properties. It is also utilized as an oxidizer in rocket propellants and in motor racing to boost engine power output.
- It is used rocket motor as an oxidizer
- It is used in the manufacturing of semiconductors
- It is used as a flavouring ingredient
- It is used in dentistry
- It is used to manufacture chemicals.
Health Hazards
Humphry Davy invented the term “laughing gas” to describe the euphoric feelings it produces when inhaled, a feature that has led to its recreational use as a dissociative anesthetic. It is included on the World Health Organization’s List of Essential Medicines, which includes the safest and most effective medicines required in a health-care system.