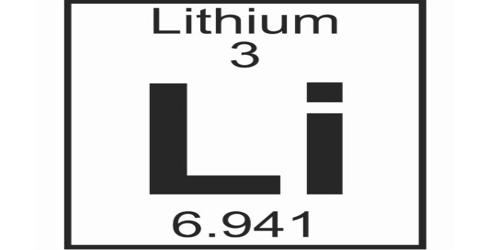
Lithium (Li) is a chemical element of Group 1 (Ia) in the periodic table, the alkali metal group, lightest of the solid elements. It is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. It has the lowest density of all metals. It reacts vigorously with water. Under standard conditions, it is the lightest metal and the lightest solid element. Like all alkali metals, lithium is highly reactive and flammable and must be stored in mineral oil. The alkali metals include sodium, potassium, rubidium, cesium, and rancium. Lithium is also the least dense of all metals. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium.
- atomic number: 3
- atomic weight: 6.941
- melting point: 180.5 °C (356.9 °F)
- boiling point: 1,342 °C (2,448 °F)
- specific gravity: 0.534 at 20 °C (68 °F)
- oxidation state: +1
Lithium was discovered from a mineral, while other common alkali metals were discovered from plant material. Discovered in 1817 by Swedish chemist Johan August Arfwedson in the mineral petalite, lithium is also found in brine deposits and as salts in mineral springs; its concentration in seawater is 0.1 part per million (ppm). Lithium’s hardness on the Mohs scale is 0.6. The Mohs scale is a way of expressing the hardness of a material.
The nucleus of the lithium atom verges on instability since the two stable lithium isotopes found in nature have among the lowest binding energies per nucleon of all stable nuclides. Because of its relative nuclear instability, lithium is less common in the solar system than 25 of the first 32 chemical elements even though its nuclei are very light: it is an exception to the trend that heavier nuclei are less common. For related reasons, lithium has important uses in nuclear physics.
It has a melting point of 180.54°C (356.97°F) and a boiling point of about 1,335°C (2,435°F). Its density is 0.534 grams per cubic centimeter. It reacts slowly with water at room temperature and more rapidly at higher temperatures.
Uses
Lithium has a number of important and interesting uses. In recent years, it has been used to make lightweight, efficient batteries.
- The most important use of lithium is in rechargeable batteries for mobile phones, laptops, digital cameras, and electric vehicles.
- It is used for mental illnesses, including bipolar disorder, depression, and schizophrenia.
- It supplements may also be used for other conditions, but there is limited scientific evidence to support these uses.
- Its metal is made into alloys with aluminum and magnesium, improving their strength and making them lighter.
- Lithium oxide is used in special glasses and glass-ceramics.
- Lithium chloride is one of the most hygroscopic materials known and is used in air conditioning and industrial drying systems.