Potassium Carbonate
Defintion
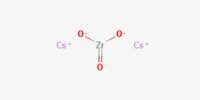
Potassium carbonate (K2CO3) is a transparent, white, deliquescent, granular powder used in making soaps. It is also called potash. It can be made as the product of potassium hydroxide’s absorbent reaction with carbon dioxide. It is deliquescent, often appearing a damp or wet solid.

Potassium carbonate is the primary component of potash and the more refined pearl ash or salts of tartar. Historically, pearl ash was created by baking potash in a kiln to remove impurities. The fine, white powder remaining was the pearl ash. The first patent issued by the US Patent Office was awarded to Samuel Hopkins in 1790 for an improved method of making potash and pearl ash. In late 18th century North America, before the development of baking powder, pearl ash was used as a leavening agent in quick breads.
It is used in fire extinguishers, to make soap, to make glass, and to soften water. It is also found in effervescent tablets. Effervescent tablets and powders are available to provide potassium when there are low levels of potassium in the blood due to inadequate diet, nausea and vomiting, diarrhea or use of certain medications such as corticosteroids or diuretics. They dissolve quickly, are stable, convenient and easy to carry.
Production and Properties of Potassium Carbonate
Potassium carbonate is a white salt, soluble in water (insoluble in alcohol) which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide’s absorbent reaction with carbon dioxide. It is deliquescent, often appearing a damp or wet solid. The production of pure potassium carbonate from technically pure caustic potash has hitherto occasioned great difliculty, and has only been successfully obtained by means of diiiicult and expensive processes. It is true that, by passing carbonic acid into a caustic potash solution until it is saturated, a complete conversion into potassium carbonate is easily obtained; the resulting potassium carbonate solution, however, contains all the impurities of the raw material from which it is produced. Conseqgently the separation of pure potassium car onatei’rom this solution is not possible without further treatment.
Potassium is excellent for heart health, If a person does not have enough potassium in the body, a condition known as hypokalemia, negative symptoms can occur. These include fatigue, muscle cramping, constipation, bloating, muscle paralysis and potentially life-threatening heart rhythms, according to the Linus Pauling Institute. Taking potassium bicarbonate can help to reduce these symptoms. Potassium bicarbonate also can lower blood pressure and reduce the risk of developing kidney stones.

Applications of Potassium Carbonate
Potassium carbonate is an important raw material in basic inorganic chemical industry, medicine industry and light industry. It has been mainly used in production of optical glass, electrode tube, and TV tube, bulb, printing items, dye, ink, photography items, sodium metasilicate, polyester powder, plating, leather, ceramic building materials, crystal, potash soap and drugs.
Besides, it also can be used as gas adsorbent, dry powder and rubber protective agent. It can be used for removal of carbon dioxide in chemical fertilizer syngas. It also can be used as a potassic fertilizer. Potassium carbonate also extends its application in the field of detergent builder, gourmet and food.
Reference: