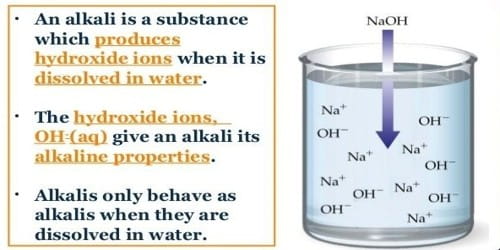
Alkali is a basic hydroxide or ionic salt which is soluble in water. In chemistry, an alkali is an aqueous (from water) solution with a pH value of more than 7. The word ‘Alkali’ comes from the Arabic ‘qali’ meaning ‘from the ashes’ since ashes mixed with water used as cleaning products (such as soaps) are made of alkali materials. The term alkali is also applied to the soluble hydroxides of such alkaline-earth metals as calcium, strontium, and barium and also to ammonium hydroxide. It also can be defined as a base that dissolves in water.
An alkali is where a base is dissolved in water. Often it is the salt of an Alkali metal. All bases are not alkali. An alkali is the opposite of acid and can be neutralized (brought down to pH 7) by adding acid. Alkalis form chemical salts when they are combined with acids.
Characteristics
- It feels soapy, or slippery feel.
- It is corrosive (it can burn your skin away)
- The higher the number is over 7 on the pH scale the stronger the alkali is.
- They have a bitter taste
- Turns red litmus paper blue
- Highly soluble in water.
- Can conduct electricity due to the presence of mobile ions
- Is blue or purple on a universal indicator
Strength
Like acids, alkalis can be weak or strong, depending on the nature and the concentration of the ionic salt composing it. The strength of alkali can be found using a universal indicator. Also like acids, the strength of an alkali is rated using the pH scale. The pH of a substance depends on how atoms are arranged and combined in the substance. When a substance has more hydroxide atoms (OH-), it is an alkali, or basic.
For example, soap and toothpaste contain weak alkalis, while cleaning products often contain strong ones.
- Examples of common Alkalis
- Sodium hydroxide, NaOH
- Potassium hydroxide, KOH
- Calcium hydroxide, Ca(OH)2
- Aqueous ammonia, NH3 (aq)
Uses of common Alkalis
- Sodium hydroxide is used to make paper, detergents, and soap.
- Potassium hydroxide is used in farming to make acidic soil more alkaline so that plants will grow better in it, and is also used as the electrolyte in alkaline, Ni-Cd, and Ni-MH batteries.
- Calcium hydroxide is used to neutralize acidic soil.
- Ammonium hydroxide is used as a cleansing agent.
- The manufacture of industrial alkali usually refers to the production of soda ash and caustic soda.
Information Source: