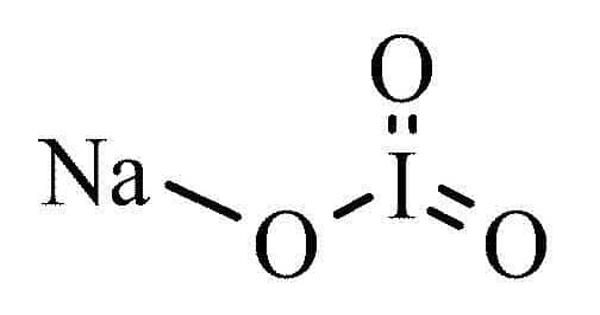
Sodium iodate (NaIO3) is an organic molecular entity. It is the sodium salt of iodic acid. It is a dough conditioner used to strengthen doughs. It is commonly found as a white crystalline powder and has the following physical properties. It is an oxidizing agent, and as such it can cause fires upon contact with combustible materials or reducing agents. It is commonly used in leavened products such as bread, rolls, and sweet rolls.
Properties
It is commonly found as a white crystalline powder and has the following physical properties.
- Molecular Weight: 197.89
- Appearance: powder
- Melting Point: N/A
- Boiling Point: N/A
- Density: 3.56 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 197.878982

Preparation
Sodium iodate is an oxidizing agent and as such it can cause fires if in contact with combustible materials or reducing agents. It can be prepared by reacting a sodium-containing base such as sodium hydroxide with iodic acid, for example:
HIO3 + NaOH → NaIO3 + H2O
It can also be prepared by adding iodine to a hot, concentrated solution of sodium hydroxide or sodium carbonate:
3 I2 + 6 NaOH → NaIO3 + 5 NaI + 3 H2O
Conditions/substances to avoid are: heat, shock, friction, combustible materials, reducing materials, aluminum, organic compounds, carbon, hydrogen peroxide, sulfides.
Reactions
Sodium iodate can be oxidized to sodium periodate in water solutions by hypochlorites or other strong oxidizing agents:
NaIO3 + NaOCl → NaIO4 + NaCl
Sodium iodate impurity in mother solution is reduced by sodium sulfite, and sodium iodate, contained in the solid phase, is isolated after its repulping at mass ratio solid: liquid 1:1.8-3.4 at 15-40 deg. The solution formed is returned to the stage of iodine interaction with NaOH.
Uses
The main use of sodium iodate in everyday life is in iodised salt. It is a source of iodine, an essential nutrient for the synthesis of thyroid hormones (thyroxine and triiodothyronine) involved in controlling basal metabolic rate and regular growth and development. The other compounds which are used in iodised table salt are potassium iodate, potassium iodide, and sodium iodide. Sodium iodate comprises 15 to 50 mg per kilogram of applicable salt.
Sodium iodate is also used as a dough conditioner to strengthen the dough. As an oxidizing agent, sodium iodate is considered a fast oxidizer which requires care when developing formulations and processing methods.
Safety
Conditions/substances to avoid are: heat, shock, friction, combustible materials, reducing materials, aluminium, organic compounds, carbon, hydrogen peroxide, sulfides.
Information Source: