By illuminating the process by which cyanobacteria (Synechocystis sp. PCC 6803) manufacture D-lactate, a research team lead by Kobe University has demonstrated that malic enzyme enhances this creation. Then, through genetic engineering, they were able to change the D-lactate synthesis route and produce the most D-lactate (26.6g/L) ever straight from CO2 and light.
This accomplishment is anticipated to aid in the creation of significant process technologies for the production of polylactic acid, which is utilized to create biodegradable plastics. This might contribute to the realization of the idea of a low-carbon, sustainable society.
The research group consisted of Professor HASUNUMA Tomohisa (of Kobe University’s Engineering Biology Research Center), Project Associate Professor HIDESE Ryota (of Kobe University’s Graduate School of Science, Technology and Innovation) and Associate Professor OSANAI Takashi (of Meiji University’s School of Agriculture).
On January 31, 2020, the findings of this study were made available online in the peer-reviewed journal ACS Synthetic Biology.
It is crucial for the environment and the sustainability of resources to use bioproduction to create flexible chemical compounds and functional raw materials, which are often derived from oil.
Microbe-based bioproduction techniques have become more popular recently. Microalgae are one of these bacteria. Utilizing CO2 and sunlight, it is possible to create a variety of useful materials from microalgae, including oils and pigments.
A fast-growing variety of microalgae called cyanobacteria is simple to genetically alter. Although cyanobacteria have been utilized in the past to manufacture D-lactate, the poor yield has been a barrier to their practical usage.
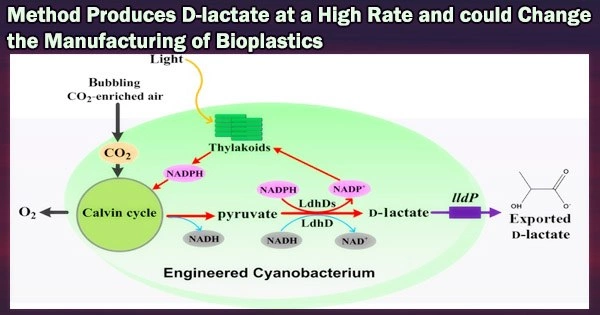
Through photosynthesis, cyanobacteria convert CO2 into the sugar glycogen. In a dark, oxygen-free environment, cyanobacteria that have stored glycogen inside their cells begin to break down the glycogen and release organic acids (such as succinic and lactic) into the growth medium.
Pyruvate production must rise in order for cyanobacteria to produce D-lactate. This research team found that the formation of D-lactate depends on the malic enzyme, which transforms malic acid into pyruvate. To provide light on the process behind the formation of D-lactate, they used dynamic metabolomics.
Through this investigation, they were able to determine that when too much malic enzyme is created within the cells, both the pathway to produce pyruvate from glycogen and the conversion of malic acid into pyruvate are active. D-lactate dehydrogenase biosynthesizes D-lactate from pyruvate.
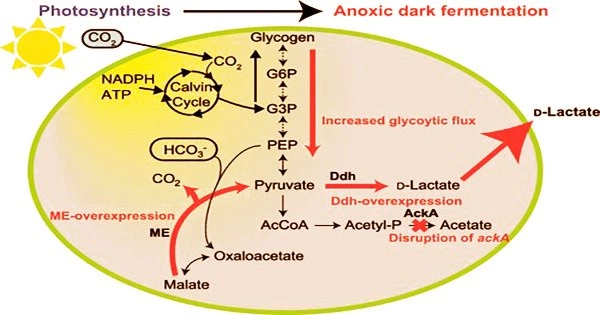
The research team was able to genetically modify the D-lactate dehydrogenase to maximize its performance, producing 26.6 g/L of D-lactate with a conversion rate of 94.3% from the accumulated glycogen.
This study represents a significant step forward in the creation of a commercial technique to create D-lactate from CO2. Through investigation of cultivation conditions and improvement of metabolic pathways, the group hopes to continue increasing D-lactate production.
D-lactate, which can be utilized as a raw material in the creation of stereocomplex PLA, a biodegradable plastic, has a sizable market. On the other hand, to make biologically generating D-lactate utilizing bacteria practical, high purity and productivity are needed.
There are bioproduction techniques that employ heterotrophic microorganisms like E. coli, however these rely on corn or sugarcane’s sugar (glucose) as a source of energy for production. Growing these plants for bioproduction has a number of negative effects, including rivalry with food sources, usage of freshwater resources and arable land, and contribution to environmental degradation (for example, deforestation).
On the other hand, because it can transform CO2 fixed by photosynthesis into a variety of target molecules, cyanobacteria are the perfect bacterium for creating useful things. Cyanobacteria can also flourish in intense light since they have a considerably larger capacity for photosynthesizing than do plants. It can be grown in seawater and doesn’t need soil for many types. As a result, it is envisaged that cyanobacteria, which just need sunlight, CO2, and seawater, will provide the foundation for bioproduction.
The possibility of employing cyanobacteria to produce D-lactate is well known, and efforts have been undertaken to increase D-lactate production through genetic engineering. Low concentrations of this target molecule are, however, produced since practically all systems that create D-lactate are linked to propagation through photosynthesis. This is due to the lack of knowledge regarding the process underlying D-lactate synthesis in cyanobacteria.
Researchers can now detect and calculate the many substances found inside cells thanks to metabolome analysis tools. In order to track the quantity of drugs metabolized over time, this research team created “Dynamic Metabolomics.”
One of the most often studied cyanobacteria in the world is Synechocystis sp. PCC 6803, which was employed in this investigation. Because it is simple to genetically manipulate and develops quickly, it serves as a model organism for the synthesis of photosynthate. Dynamic metabolomics was previously used by this team to demonstrate that succinic acid is mostly generated by malic acid in Synechocystis sp. PCC 6803.
The malic enzyme, which changes malic acid into pyruvate, was the subject of the current investigation. First, they used dynamic metabolomics to clarify how the malic enzyme affected Synechocystis sp. PCC 6803’s metabolism. The next goal was to use metabolic engineering to boost D-lactate synthesis.
Research Methodology
Two types of cell were created in order to comprehensively investigate the mechanism behind D-lactate production: 1. Cells which had no malic enzyme function and 2. Cells in which this function was optimized, leading to an overexpression of malic enzyme.
The metabolic variance between these two cells was examined using dynamic metabolomics. It was discovered that when the amount of malic acid in the cells was low, more pyruvate was created from glycogen.
The study team also genetically tweaked the malic enzyme to make cells that overexpress D-lactate dehydrogenase and improve that enzyme’s ability to convert pyruvate into D-lactate. The team also genetically altered the cells to eliminate the acetate kinase enzyme in an effort to reduce the formation of byproduct acids.
The modified Synechocystis sp. PCC 6803 was then cultivated in a dark anoxic environment (fermentation conditions). Under these conditions, the cells reached the optimum density. This research group far exceeded the world’s previous highest yield of D-lactate (10.7 g/L), by producing 26.6 g/L at a rate of 0.185 g/L/h. This discovery may help develop a low-cost method for producing large amounts of D-lactate.
Further Research
Although cyanobacteria can be used to manufacture a wide range of adaptable chemical compounds and useful raw materials, this technology has not yet reached a stage where it can be applied on a large-scale industrial basis. The main issue is that, when cyanobacteria are used instead of heterotrophic microorganisms, smaller concentrations of the target chemical are produced.
According to recent research, it is very efficient to assess Synechocystis function using dynamic metabolomics analysis. Based on the findings of the dynamic metabolomics, this team genetically altered the Synechocystis’ metabolism in order to maximize performance.
`1It is envisaged that metabolic engineering and dynamic metabolomics can help cyanobacteria produce more photosynthetic material, advancing the goal of a sustainable, low-carbon civilization.