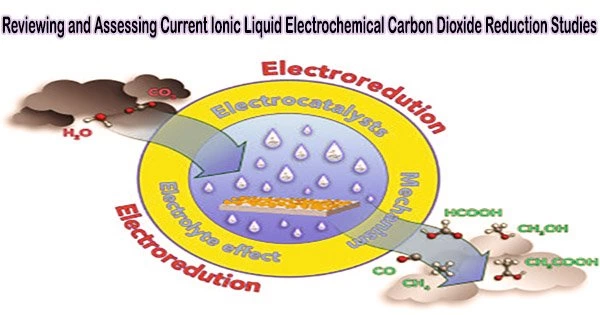
Electrochemical CO2 reduction (CO2R) to the added-value carbon-based chemicals could be used to address the rising CO2 emission, which is the main cause of many environmental concerns. Ionic liquids (ILs) have been extensively researched to support CO2R as electrolytes and co-catalysts because of their distinctive advantages.
The C1 products of CO2R, which include CO, CH3OH, CH4, and syngas, only contain one carbon atom and are therefore easier to produce than other CO2R products. Numerous related experimental research and reviews have been published over the past 12 years to support the development of CO2R-to-C1 products, and in these recent years, significant progress has been made.
However, to the best of our knowledge, no work has been conducted to discuss and systematically compare the economic benefits of different C1 products (CO, CH3OH, CH4, H2/CO(1:1) and H2/CO(2:1)) focusing on the IL-based electrolyte systems, as well as analyzing environmental impacts, to give guidance for realizing the commercialization of CO2R technology in the near future.
The research on CO2R-to-C1 products based on IL-based electrolytes has been summarized and updated here. A team of scientists has also thoroughly assessed the economic benefit and environmental impact of current and potential future technologies. Their work was published in Industrial Chemistry & Materials.
In this review, our main goal is to provide readers with intuitionistic insight on the commercial potential of CO2R-to-C1 products with ILs as the electrolyte based on the state-of-the-art and future scenarios from both economic and environmental aspects.
Professor Xiaoyan Ji
“As the rapid development of CO2R with ILs-based electrolyte in the lab, it is necessary to have a clear insight on their commercial value when scale-up it to an industrial scale,” said Xiaoyan Ji, a professor at Luleå University of Technology.
“In this review, we summarized the experimental achievements of CO2R-to-C1 products using IL-based electrolyte, evaluated their performances from both economic and environmental aspects based the state-of-the-art technology and those with improved performance in the near future, and identified their potential for commercialization. We also put forward the strategies to boost the performance and profits for CO2R-to-C1 product with ILs as the electrolyte in the future.”
Due to its moderate conditions, ease controllability, and potential for the conversion of CO2 to value-added compounds, CO2R is one of the most promising processes. Additionally, its power source can be combined with renewable energy sources like solar, wind, and hydropower.
There are three main parameters to evaluate the performance of CO2R, including current density, Faradaic efficiency (FE), and cell voltage, which can be improved through designing and optimizing electrocatalysts and electrolytes.
ILs can offer a low overpotential and a high current density, which can increase the product selectivity for CO2R. They can also do this thanks to their adjustable architectures and characteristics, large electrochemical windows, and high electrical conductivities. Significantly, ILs can effectively inhibit the hydrogen evolution reaction (HER), which is a competitive reaction of CO2R.
“CO is the only profitable product among the studied C1 products, while the total production costs (TPC) of other products are too high to be profitable, especially for CH4 and H2/CO(2:1),” Ji said.
“This phenomenon is consistent with the performance of CO2R for each product. The current density and FE of CO are as high as 182.2 mA cm-2 and 99.7%, respectively. However, for CH4 and H2/CO(2:1), the current densities are as low as 25.6 and 11.4 mA cm-2, respectively. Additionally, with the improved performance of CO2R, CH3OH and H2/CO(1:1) can be profitable in the near future. While it is difficult for CH4 and H2/CO(2:1) to be profitable even under the most ideal scenario, partly due to the low market price. On the other side, the formation of CH4 requires transferring the largest number of electrons (8e-) among the studied C1 products.”
“CO2R-to-CH4 is the most environmentally friendly pathway compared to others,” added Xiangping Zhang, a professor at the Institute of Process Engineering, Chinese Academy of Sciences.
“While, considering both economic and environmental aspects, CO is the most attractive product. For other C1 products, further improvement of CO2R or the development of more advanced electrolyzers are required to realize their commercial value,” Zhang said.
Furthermore, ILs should be further exploited in the future CO2R as follows: (1) designing more effective and acceptable CO2R electrolytes is made possible by the customizable feature of ILs in their structure and properties; (2) by integrating additional solvents and electrolytes, the ability of ILs to dissolve a variety of solvents and electrolytes can enhance CO2R performance; (3) the cleaner ILs can be designed and synthesized applying into CO2R to mitigate the environmental burden; (4) in addition to serving as electrolytes, ILs can act as a co-catalyst or modifier for a catalyst that performs well.
“In this review, our main goal is to provide readers with intuitionistic insight on the commercial potential of CO2R-to-C1 products with ILs as the electrolyte based on the state-of-the-art and future scenarios from both economic and environmental aspects,” said Ji.