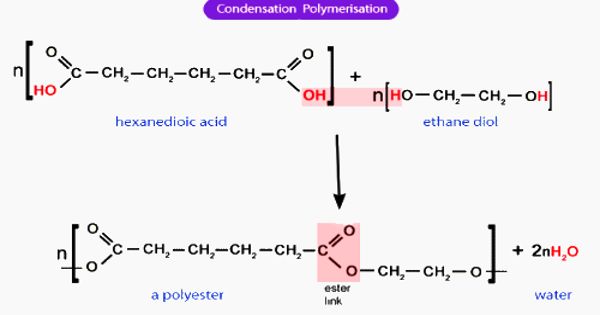
Condensation polymerization is a form of step-growth polymerization in which monomers and/or oligomers react with each other to form larger structural units while releasing smaller molecules as a byproduct such as water or methanol. These are any kind of polymers formed through a condensation reaction—where molecules join together—losing small molecules as by-products such as water or methanol. A large number of important and useful polymeric materials are not formed by addition polymerization but precede instead by conventional functional group transformations of polyfunctional reactants.
Condensation polymer is a form of a step-growth polymerization where smaller molecules or monomers react with each other to form larger structural units (usually polymers) while releasing by products such as water or methanol molecule.
Condensation polymers are formed by polycondensation when the polymer is formed by condensation reactions between species of all degrees of polymerization, or by condensative chain polymerization when the polymer is formed by sequential addition (by condensation reaction) of monomers to an active site in a chain reaction. Examples of naturally occurring condensation polymers are cellulose, starch, the polypeptide chains of proteins, and poly (β-hydroxybutyric acid), a polyester synthesized in large quantity by certain soil and water bacteria. A well-known example of a condensation reaction is the esterification of carboxylic acids with alcohols.
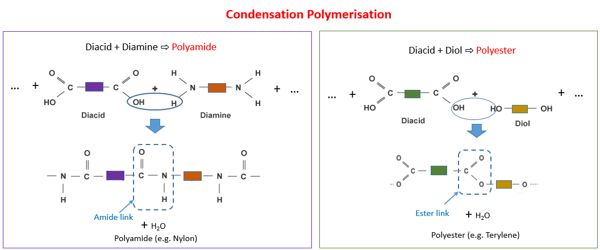
Condensation polymerization requires that the monomers possess two or more kinds of functional groups that are able to react with each other in such a way that parts of these groups combine to form a small molecule (often H2O) which is eliminated from the two pieces. The main alternative forms of polymerization are chain polymerization and polyaddition, both of which give addition polymers. If both moieties are difunctional, the condensation product is a linear polymer, and if at least one of the moieties is tri- or tetra-functional, the resulting polymer is a crosslinked polymer.
Characteristics of Condensation Polymerization:
- The molecules should have one or two functional groups (like alcohol, amine, or carboxylic acid groups).
- Smaller molecules usually combine to form larger molecules.
- The mixed properties of both the molecules or functional groups are taken into consideration.
- A linear polymer is obtained as the condensation product when both functional groups are difunctional.
Condensation polymerization is a form of step-growth polymerization. Condensation polymers form more slowly than addition polymers, often requiring heat, and they are generally lower in molecular weight. Linear polymers are produced from bifunctional monomers, i.e. compounds with two reactive end groups. The terminal functional groups on a chain remain active so that groups of shorter chains combine into longer chains in the late stages of polymerization. Common condensation polymers include polyamides, polyacetals, and proteins.
Information Source: