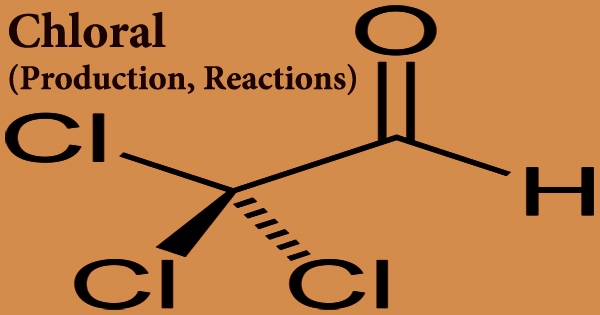
Chloral, sometimes referred to as trichloroacetaldehyde or trichloroethanal, is a colorless liquid aldehyde produced by chlorinating ethane. It was employed in the production of the pesticide DDT. Cl3CCHO is the formula for this chemical compound. This aldehyde is a colorless, oily liquid that may be dissolved in a variety of solvents. It’s a colorless, oily liquid with a strong odor that interacts with water and is denser than water. Contact irritates the skin, eyes, and mucous membranes; ingestion and inhalation are hazardous.
Chloral interacts with water to create chloral hydrate, a sedative and hypnotic chemical that was previously widely used. 2,2,2- trichloroethanediol (chloral hydrate, CCl3CH(OH)2) can be obtained by hydrolyzing it. The majority of compounds with two –OH groups on the same carbon atom are inherently unstable. It’s an organochlorine chemical made composed of acetaldehyde with chloro groups replacing all of the methyl hydrogens. It functions as a metabolite in mice.
The action of the three chlorine atoms, on the other hand, stabilizes the molecule in this situation. As a sedative, it is utilized. In 1832, the German scientist Justus von Liebig synthesized and called chloral for the first time. Liebig used dry chlorine gas to cure anhydrous ethanol. It’s an aldehyde and an organochlorine chemical. It’s made up of acetaldehyde. Chloral hydrate is manufactured commercially by chlorinating acetaldehyde in the presence of hydrochloric acid to create chloral. Ethanol may be utilized as a feedstock as well. Antimony trichloride catalyzes this process:
H3CCHO + 3 Cl2 + H2O → Cl3CCH(OH)2 + 3 HCl
The chloral hydrate is extracted from the reaction mixture by distillation. The distillate is next dehydrated with strong sulfuric acid, followed by the removal of the heavier acid layer (which contains the water):
Cl3CCH(OH)2 → Cl3CCHO + H2O
Fractional distillation is used to purify the final product. Some chlorinated water contains trace quantities of chloral hydrate. When chloral is exposed to moisture and light, it becomes unstable. It’s water-soluble. Chloral hydrate is formed when it interacts with water. Under the action of light and in the presence of sulfuric acid, it polymerizes to produce metachloral, a white solid trimer. Chloral tends to form adducts with water (to give chloral hydrate) and alcohols.
Chloral is significant as a building component in the production of DDT, despite its proclivity for hydrating. Chloral is treated with chlorobenzene in the presence of a catalytic quantity of sulfuric acid for this purpose:
Cl3CCHO + 2 C6H5Cl → Cl3CCH(C6H4Cl)2 + H2O
Othmar Zeidler first reported this response in 1874. Chloral is also used to make the herbicide methoxychlor. DDT, methoxychlor, DDVP, naled, trichlorfon, and TCA are all insecticides that include chloral as an intermediate. Chloral was the most abundant metabolite found, and its levels varied by species and sex; trichloroethanol levels were similarly variable by species and sex, but never topped 15% of total metabolites.
Chloral is also used to make chloral hydrate; it was once employed as a medicinal drug having hypnotic, sedative, and narcotic properties before barbiturates were introduced. Chloral is converted to chloroform Cl3CH and sodium formate HCOONa when it is treated with sodium hydroxide.
Cl3CCHO + NaOH → Cl3CH + HCOONa
Trichloroethanol, which is generated in the body from chloral, is easily decreased. Chlorination of ethyl alcohol, sulfuric acid addition, and subsequent distillation Because the former hydrates quickly, chloral and chloral hydrate have the same biological characteristics. Water is the major contaminant in technical-grade chloral, which ranges in purity from 94 to 99 percent by weight. Chloroform, hydrogen chloride, dichloroacetaldehyde, and phosgene are some of the other contaminants that can be found.
On the gram scale, chloral hydrate was frequently given to patients with no long-term consequences. Incineration after combining with another combustible fuel; full combustion is required to avoid the production of phosgene; an acid scrubber is required to remove the halo acids generated. The chloral is treated for 2 minutes with 1N sodium hydroxide, which breaks down the chloral into chloroform and sodium formate, and the surplus alkali is then titrated with acid. Alternatively, chloral is reacted with quinaldine ethyl iodide to produce a blue cyanine dye, the amount of which is determined using spectrophotometry.
Information Sources: