Higher alcohols (C2+ alcohols), which are crucial basic materials, have been used as the building blocks of priceless goods. They are frequently used in a number of industries, including those that produce fine chemicals, food, pharmaceuticals, and energy.
Direct synthesis of higher alcohols from syngas has emerged as a viable and sustainable technique due to the steady depletion of petroleum supplies and its abundant supply of raw materials and high atomic utilization. However, the low yield of higher alcohols restricted industrial application.
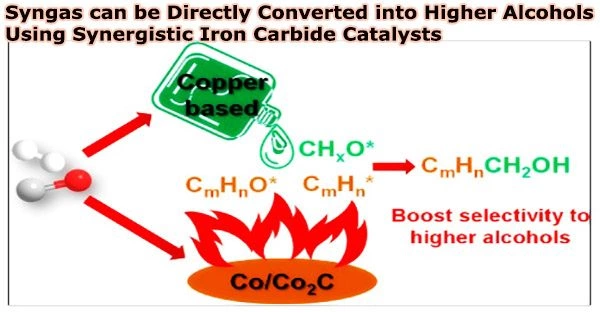
Recently, a research team led by Prof. Sun Jian and Prof. Ge Qingjie from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has realized the direct synthesis of higher alcohols from syngas and achieved the highly selective production of higher alcohols from CO hydrogenation over synergistic iron carbides catalysts.
This study extends the understanding of the important role of Ca and provides a new strategy for the design of catalysts in CO hydrogenation to higher alcohols.
Professor Sun Jian
This study was published in Chem Catalysis.
“The proposed synergistic Ca-Fe series catalysts could realize a oxygenate selectivity of 69.7 wt% under mild conditions, alongside the alcohols fraction of 86.4 % in oxygenates,” said Prof. Sun. “The total oxygenates and alcohols selectivity outperformed the results of previously reported catalysts.”
The researchers discovered through several characterizations that the Ca loading allowed the Ca-Fe catalyst to have a high surface iron carbide concentration and a suitable Fe2C/(Fe5C2+Fe3C) ratio. A synergistic impact with Fe2C (CO non-dissociative sites) could help the Fe5C2 and Fe3C as CO dissociative sites to create greater alcohols.
Therefore, the proper Fe2C/(Fe5C2+Fe3C) ratio facilitated the balance of the CO dissociative and non-dissociative abilities, and boosted the better cooperation of *CO and *CHx to form the *CHx-*CO species, further promoting the higher alcohols formation.
“This study extends the understanding of the important role of Ca and provides a new strategy for the design of catalysts in CO hydrogenation to higher alcohols,” said Prof. Sun.