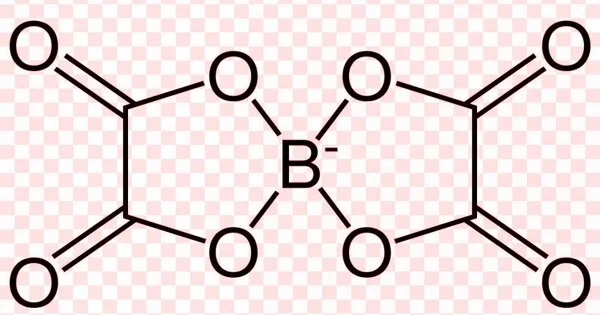
Borate oxalates are chemical compounds that contain the anions borate and oxalate. When the oxalate group is bound to the borate via oxygen, a more condensed anion that balances fewer cations is formed. These are known as boro-oxalates, bis(oxalato)borates, oxalatoborates, and oxalate borates. Oxalatoborates are heterocyclic compounds with the ring -O-B-O-. Bis(oxalato)borates are spiro compounds that have rings that are connected at the boron atom.
Oxalatoborates are used in lithium-ion battery electrolytes and supercapacitors for research. Boron is a one-of-a-kind and exciting element. It has served as a constant challenge and stimulus not only to preparative chemists and theorists, but also to industrial chemists and technologists over the years.
Properties
When heated to around 320 °C, oxalaotoborates decompose to produce a metaborate, carbon monoxide, and carbon dioxide.
Borates are a common class of flame retardants that can be found as boric acid and in a variety of salts, particularly sodium borate (borax) and zinc borates. Boric acid (H3BO3) and its sodium salt, borax, were among the first flame retardants to be used. There are both primary flame retardants and synergists in the boric acid/borate family.
Production
Boric acid, oxalic acid, and one of a metal oxalate, a metal carbonate, or an amine were heated in boiling benzene to produce oxyalatoborates. Boric acid and ammonium borate types primarily function through the solid phase charring process and are most effective in easily charred cellulosic materials. Zinc borates are multifunctional flame retardants in which both the zinc and borate constituents play a role. Their functions include the endothermic release of water, some gas phase activity in the presence of halogens, and the production of a strong boron-based char under fire conditions.
Borates constitute a class of about four dozen minerals that almost rival silicates in diversity and complexity. The basic building block of many borates is a boron surrounded by three oxygen atoms; however, boron may also be surrounded by four oxygens in a tetrahedral configuration like that of the silicates.
Applications
Borates are used in both halogen-containing and halogen-free polymers as flame retardants, smoke suppressants, afterglow suppressants, and antitracking agents. Boron compounds such as boric acid (BA) and sodium borates are well-known cellulosic flame retardants. However, the use of boron compounds as flame retardants in plastics and rubbers, such as zinc borates, ammonium pentaborate, melamine borate, boron phosphate, metal borophosphate, and borosiloxane, has only recently become popular.