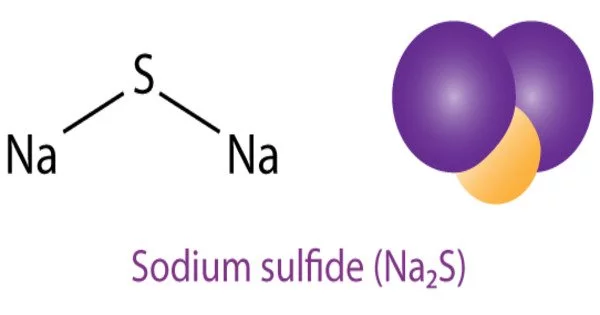
Sodium sulfide is an inorganic compound with the chemical formula Na2S. It is a colorless to yellowish solid that is soluble in water and is commonly used in the production of chemicals, dyes, and detergents. It is also used in the leather industry for dehairing hides and in the production of paper and pulp.
Both the anhydrous and hydrated salts are colorless solids in pure crystalline form, though technical grades of sodium sulfide are generally yellow to brick red due to the presence of polysulfides and are commonly supplied as a crystalline mass, flake form, or fused solid. They are water-soluble and produce highly alkaline solutions. When Na2S and its hydrates are exposed to moist air, they emit hydrogen sulfide, an extremely toxic, flammable, and corrosive gas that smells like rotten eggs.
Properties
In its solid form, sodium sulfide appears as a white or yellowish powder. It is hygroscopic, which means it readily absorbs moisture from the air, and it has a strong odor of hydrogen sulfide, which is a toxic gas. Sodium sulfide can be corrosive and should be handled with care.
- Chemical formula: Na2S
- Molar mass: 78.0452 g/mol (anhydrous); 240.18 g/mol (nonahydrate)
- Appearance: colorless, hygroscopic solid
- Odor: none
- Density: 1.856 g/cm3 (anhydrous); 1.43 g/cm3 (nonohydrate)
- Melting point 1,176 °C (2,149 °F; 1,449 K) (anhydrous); 100 °C (pentahydrate)
- Solubility in water: 12.4 g/100 mL (0 °C); 39 g/100 mL (50 °C)(hydrolyses)
- Solubility: insoluble in ether; slightly soluble in alcohol
Production
Industrially Na2S is produced by carbothermic reduction of sodium sulfate often using coal:
Na2SO4 + 2 C → Na2S + 2 CO2
In the laboratory, the salt can be prepared by reduction of sulfur with sodium in anhydrous ammonia, or by sodium in dry THF with a catalytic amount of naphthalene (forming sodium naphthalenide):
2 Na + S → Na2S
Application
Sodium sulfide is often used as a reducing agent in chemical reactions. It is also used as a source of sulfide ions, which can be used in analytical chemistry to precipitate metals from solutions. In the mining industry, it is used to separate ores containing precious metals such as gold and silver from their ores.
Sodium sulfide has several important applications in the production of polymers, such as the production of rayon fibers and cellophane. It is also used in the production of rubber chemicals, as a pulp processing aid, and as a water treatment chemical to remove heavy metals from wastewater.