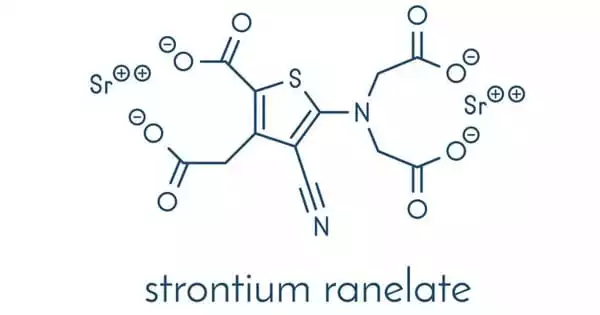
Strontium is a silvery metal that exists in nature as a non-radioactive element. The bones contain approximately 99 percent of the strontium in the human body. Strontium ranelate, a strontium(II) salt of ranelic acid, is an osteoporosis medication marketed by Servier under the brand names Protelos or Protos. According to research, it can also slow the progression of knee osteoarthritis. The drug is unique in that it both increases new bone formation by osteoblasts and decreases bone resorption by osteoclasts.
Strontium ranelate is a divalent strontium salt of ranelic acid that can increase bone formation while decreasing bone resorption, uncoupling, and rebalancing bone turnover in favor of bone formation. In patients with postmenopausal osteoporosis, the drug is effective in lowering the risk of fractures, including both vertebral and nonvertebral fractures.
Strontium ranelate is a drug used to treat osteoporosis. It comes in the form of a white to light yellow powder or crystalline powder. It has no odor and is slightly soluble in water; however, it is almost insoluble in ethanol and easily soluble in dilute hydrochloric acid. It was first studied and developed by the French Servier Company, and it first went on sale in Ireland in November 2004, followed by the United Kingdom in December of the same year. Fujisawa Pharmaceutical Company of Japan has the authorization for the development, production, and marketing of this product in Japan. It is primarily used in the treatment and prevention of osteoporosis in postmenopausal women, significantly lowering the risk of vertebral fractures and hip fractures.

Strontium ranelate has a dual pharmacological inhibitory effect, inhibiting bone absorption while also encouraging bone formation. On the one hand, it can increase the synthesis of collagen and non-collagen proteins in osteoblast-enriched cells and promote osteoblast-mediated bone formation mediated by osteoblasts by enhancing pre-osteoblast proliferation. On the other hand, by decreasing osteoclast differentiation and reabsorbing activity, as well as further reducing bone absorption, it achieves a rebalance of bone turnover, thereby increasing bone formation.
Uses
Strontium ranelate is a prescription drug that is approved in over 70 countries for the treatment of postmenopausal osteoporosis in order to reduce the risk of vertebral and hip fractures. The FDA has not approved strontium ranelate for use in the United States. Strontium ranelate is a medicine prescribed by the National Health Service in the United Kingdom for the treatment of postmenopausal osteoporosis.
In the year 2000, two large phases III clinical trials, SOTI (Spinal Osteoporosis Therapeutic Intervention) and TROPOS (Treatment of Peripheral Osteoporosis), were launched to investigate the efficacy of strontium ranelate in reducing vertebral fractures and peripheral fractures, including hip fractures. Strontium ranelate showed a significant reduction in vertebral fractures (41% reduction) and hip fractures (36% reduction) compared to patients treated with placebo over a three-year period, according to results published in 2004.
The efficacy was maintained over a five-year period of data. The 5-year data confirmed that strontium ranelate can significantly reduce vertebral fractures regardless of the risk factors of osteoporotic women. These factors include their age (70, 70–80, and >80), bone mineral density (osteoporotic and osteopenia), prevalent fractures (0, 1–2, and >2 prevalent fractures), symptomatic fractures, body mass index, and smoking.
In very old elderly and osteopenic patients, strontium ranelate has anti-fracture efficacy.