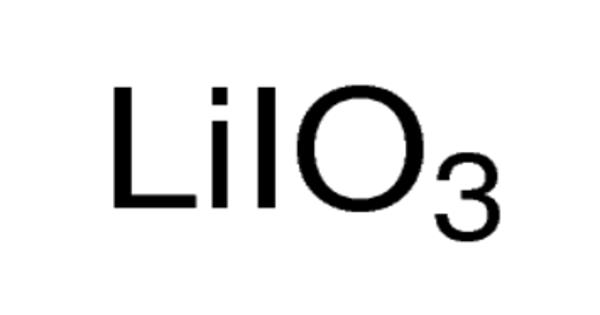Lithium iodate (LiIO3) is a negative uniaxial crystal for nonlinear, acousto-optical and piezoelectric applications. It is a compound of lithium and iodine. It has been utilized for 347 nm ruby lasers. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine. It is an inorganic compound in which lithium and iodine bond with each other to form lithium iodide. Lithium Iodate is a crystalline solid used in photo optic applications.
Properties
Mohs hardness of lithium iodate is 3.5–4. Its linear thermal expansion coefficient at 298 K (25 °C; 77 °F) is 2.8·10−5/°C (a-axis) and 4.8·10−5/°C (c-axis). Its transition to β-form begin at 50 °C (122 °F) and it is irreversible. Moreover, it can exist in different hydrate forms such as a monohydrate, dehydrate, and trihydrate.
- Molecular Weight: 181.84
- Appearance: White crystalline solid
- Melting Point: 420 °C
- Boiling Point: N/A
- Density: 4.487 g/cm3
- Solubility in H2O: 82.7 g/100 mL (25 °C)
Preparation
The preparation of lithium iodate solutions by reacting a lithium-containing substance with elemental iodine, the lithium iodide being prepared in an aprotic solvent that can be used as a solvent in a liquid electrolyte solution. In organic synthesis, LiI is useful for cleaving C-O bonds. For example, it can be used to convert methyl esters to carboxylic acids:
RCO2CH3 + LiI → RCO2Li + CH3I
Similar reactions apply to epoxides and aziridines. We can easily identify it as when it exposed to air changes its color from white to yellow sue to the formation of iodine by oxidation of iodide.
Occurrences
Generally, it is available in large volumes, high purity, nanopowder, and submicron forms. It is highly reactive that’s why it does not occur in nature freely.
Application
- Lithium iodide is used as a solid-state electrolyte for high-temperature batteries.
- The solid is used as a phosphor for neutron detection.
- It is also used, in a complex with Iodine, in the electrolyte of dye-sensitized solar cells.
- Lithium iodide was used as a radiocontrast agent for CT scans. Its use was discontinued due to renal toxicity.
Safety and Health Hazards
Inhalation, skin contact, and ingestion with it can result in severe injury or death. Burning of lithium hydroxide can produce toxic fumes that can cause irritation or corrosion. It is highly reactive and its container can explode if heated.
Information Source: