Ruthenium is one of the rarest elements in the Earth’s crust. Ruthenium is a chemical element with the symbol Ru and atomic number 44. It belongs in the platinum group of metals. It is a rare transition metal belonging to the platinum group of the periodic table. Its abundance is estimated at about 0.0004 parts per million. Like the other metals of the platinum group, ruthenium is inert to most other chemicals. This makes it one of the six least abundant elements on Earth. Ruthenium is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America.
Ruthenium is a rare transition metal belonging to the platinum group of the periodic table. It is used in electrical contact alloys and filaments, in jewelry, in pen nibs, and in instrument pivots.
Russian-born scientist of Baltic-German ancestry Karl Ernst Claus discovered the element in 1844 at Kazan State University and named ruthenium in honor of Russia. Credit for the discovery of ruthenium is often given to Polish chemist Jedrzej Sniadecki (1768-1838). Sniadecki announced the discovery of the element in 1808.
Properties
Ruthenium is a hard, silvery-white metal with a shiny surface. It is one of the most effective hardeners for platinum and palladium, and is alloyed with these metals to make electrical contacts for severe wear resistance. Its melting point is about 2,300 to 2,450°C (4,200 to 4,400°F) and its boiling point is about 3,900 to 4,150°C (7,100 to 7,500°F). Its density is 12.41 grams per cubic centimeter.
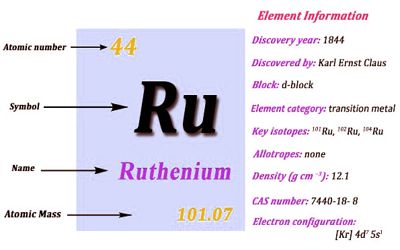
- atomic number: 44
- atomic weight: 101.07
- melting point: 2,250° C (4,082° F)
- boiling point: 3,900° C (7,052° F)
- specific gravity: 12.30 (20° C)
- valence: 1, 2, 3, 4, 5, 6, 7, 8
Occurrence
Ruthenium is found free in nature often with the other platinum group metals. As the 74th most abundant element in Earth’s crust, ruthenium is relatively rare, found in about 100 parts per trillion. Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009 to some 35.5 tonnes in 2017.
This element is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America. Small but commercially important quantities are also found in pentlandite extracted from Sudbury, Ontario, Canada, and in pyroxenite deposits in South Africa.
Uses
This makes it one of six least abundant elements on Earth. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. It is used in electrical contact alloys and filaments, in jewelry, in pen nibs, and in instrument pivots. A minor application for ruthenium is in platinum alloys and as a chemistry catalyst. A new application of ruthenium is as the capping layer for extreme ultraviolet photomasks. It has to be stored safely for at least ten years until the radioactive isotopes have decayed.