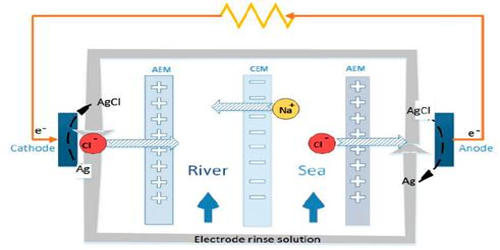
Reverse electrodialysis (RED) is a technology to generate power from mixing waters with different salinity. RED is the salinity gradient energy retrieved from the difference in the salt concentration between seawater and river water. It is based on the principle that ionic compounds such as salts dissolve in water into charged ions: equal parts positively and negatively charged ions. A method of utilizing the energy produced by this process by means of a heat engine was invented by Prof. Sidney Loeb in 1977 at the Ben-Gurion University of the Negev. A RED system uses ion-exchange membranes to separate fresh and saltwater and controls the mixing of the ions between the two solutions
In reverse electrodialysis, a salt solution and freshwater are let through a stack of alternating cation and anion exchange membranes. The chemical potential difference between salt and freshwater generates a voltage over each membrane and the total potential of the system is the sum of the potential differences overall membranes. The opposing transport of the positively and negatively charged ions creates positively and negatively charged poles, similar to a battery, and more accurately defined as an electrochemical cell. The process works through a difference in ion concentration instead of an electric field, which has implications for the type of membrane needed. Application of nanocomposite membranes containing iron (III) oxide for RED. Electrochemical characteristics were dependent on nanoparticle loadings.
In RED, as in a fuel cell, the cells are stacked. A module with a capacity of 250 kW has the size of a shipping container. In seawater, the predominant salt is sodium chloride (NaCl), which dissolves into positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). However, RED is hampered by the initial high resistance of the freshwater source, resulting in a high required membrane area (i.e., high investment costs).
When saltwater is feed in between a pair of these ion-exchange membranes then the ions will passively diffuse from the saltwater into the freshwater; however, the diffusive transport of the ions will be preferential due to the ion-exchange membranes. In the Netherlands, for example, more than 3,300 m³ freshwater runs into the sea per second on average. The membrane halves the pressure differences which results in a water column of approximately 135 meters. The energy potential is therefore e=mgΔh=3.3*106 kg/s*10 m/s2*135 meters ca.= 4.5*109 Joule per second, Power=4.5 gigawatts. This technology has grown significantly in the last decade, gaining a fast increase in its technology readiness level and presenting some interesting examples of RED pilot systems operating under very different real environments.